Vitamin A as a Transcriptional Regulator of Cardiovascular Disease
Abstract
:1. Introduction
2. Molecular Mechanisms of Vitamin A/Retinoid Signaling and Homeostasis
3. Effects of Vitamin A/Retinoids on the Formation of the Heart and Differentiation of Multipotent Cardiovascular Progenitor Cells
4. Vitamin A Signaling and Regeneration of the Myocardium
5. Population-Based Studies Implicating Vitamin A Deficiency in the Onset of CVDs
6. Effects of Vitamin A/Retinoids in the Healthy and Diseased Postnatal Myocardium
7. Effects of Vitamin A on Arterial Homeostasis
8. Effects of Vitamin A/Retinoids on Prototypical Disease Signaling Pathways
9. Antioxidant and Metabolic Properties of Vitamin A/Retinoids
10. Exogenous Retinoids or Retinoid Inhibition as Therapeutic Agents in Cardiovascular Diseases
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, L.A.; Sigman, C.C.; Andreola, F.; Ross, S.A.; Kelloff, G.J.; De Luca, L.M. Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 2000, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.S.; Pecone, D.; Pona, A.; Cline, A.; Feldman, S.R. Topical Retinoids in Acne Vulgaris: A Systematic Review. Am. J. Clin. Dermatol. 2019, 20, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dollé, P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 2002, 129, 3563–3574. [Google Scholar]
- Wilson, J.G.; Warkany, J. Aortic-arch and cardiac anomalies in the offspring of vitamin A deficient rats. Am. J. Anat. 1949, 85, 113–155. [Google Scholar] [CrossRef]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T.; et al. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef]
- Rothman, K.J.; Moore, L.L.; Singer, M.R.; Nguyen, U.S.; Mannino, S.; Milunsky, A. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 1995, 333, 1369–1373. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Mu, D.; Li, D.; Zhong, Y.; Jiang, N.; Zhang, Y.; Xia, M. Association of Serum Retinoic Acid With Risk of Mortality in Patients With Coronary Artery Disease. Circ. Res. 2016, 119, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Wirth, J.P.; Petry, N.; Tanumihardjo, S.A.; Rogers, L.M.; McLean, E.; Greig, A.; Garrett, G.S.; Klemm, R.D.; Rohner, F. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients 2017, 9, 190. [Google Scholar] [CrossRef]
- Noy, N. Retinoid-binding proteins: Mediators of retinoid action. Biochem. J. 2000, 348, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wongsiriroj, N.; Blaner, W.S. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surg. Nutr. 2014, 3, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Heyman, R.A.; Mangelsdorf, D.J.; Dyck, J.A.; Stein, R.B.; Eichele, G.; Evans, R.M.; Thaller, C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68, 397–406. [Google Scholar] [CrossRef]
- Levin, A.A.; Sturzenbecker, L.J.; Kazmer, S.; Bosakowski, T.; Huselton, C.; Allenby, G.; Speck, J.; Kratzeisen, C.; Rosenberger, M.; Lovey, A.; et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature 1992, 355, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Dollé, P. Developmental expression of retinoic acid receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002. [Google Scholar] [CrossRef] [Green Version]
- Boehm, M.F.; Zhang, L.; Zhi, L.; McClurg, M.R.; Berger, E.; Wagoner, M.; Mais, D.E.; Suto, C.M.; Davies, J.A.; Heyman, R.A.; et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J. Med. Chem. 1995, 38, 3146–3155. [Google Scholar] [CrossRef] [PubMed]
- Delescluse, C.; Cavey, M.T.; Martin, B.; Bernard, B.A.; Reichert, U.; Maignan, J.; Darmon, M.; Shroot, B. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol. Pharmacol. 1991, 40, 556–562. [Google Scholar]
- Lehmann, J.M.; Jong, L.; Fanjul, A.; Cameron, J.F.; Lu, X.P.; Haefner, P.; Dawson, M.I.; Pfahl, M. Retinoids selective for retinoid X receptor response pathways. Science 1992, 258, 1944–1946. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Duong, T.T.; Johnson, A.T.; Klein, E.S.; Wang, L.; Khalifa, B.; Chandraratna, R.A. Identification of highly potent retinoic acid receptor alpha-selective antagonists. J. Med. Chem. 1997, 40, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Thoreau, E.; Arlabosse, J.M.; Bouix-Peter, C.; Chambon, S.; Chantalat, L.; Daver, S.; Dumais, L.; Duvert, G.; Feret, A.; Ouvry, G.; et al. Structure-based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg. Med. Chem. Lett. 2018, 28, 1736–1741. [Google Scholar] [CrossRef]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [Green Version]
- Widjaja-Adhi, M.A.K.; Golczak, M. The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 23, 158571. [Google Scholar] [CrossRef]
- Palace, V.P.; Hill, M.F.; Khaper, N.; Singal, P.K. Metabolism of vitamin A in the heart increases after a myocardial infarction. Free Radic. Biol. Med. 1999, 26, 1501–1507. [Google Scholar] [CrossRef]
- Bilbija, D.; Haugen, F.; Sagave, J.; Baysa, A.; Bastani, N.; Levy, F.O.; Sirsjö, A.; Blomhoff, R.; Valen, G. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS ONE 2012, 7, e44740. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Jiang, H.; Trent, C.M.; Yuen, J.J.; Narayanasamy, S.; Curley, R.W., Jr.; Harrison, E.H.; Goldberg, I.J.; Maurer, M.S.; Blaner, W.S. Cardiac dysfunction in β-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1675–H1684. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Xu, J.; Molkentin, J.D. Re-employment of developmental transcription factors in adult heart disease. Semin. Cell Dev. Biol. 2007, 18, 117–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegan, B.R.; Feldman, J.L.; Begemann, G.; Ingham, P.W.; Yelon, D. Retinoic acid signaling restricts the cardiac progenitor pool. Science 2005, 307, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Dollé, P.; Ryckebüsch, L.; Noseda, M.; Zaffran, S.; Schneider, M.D.; Niederreither, K. Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 9234–9239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryckebusch, L.; Wang, Z.; Bertrand, N.; Lin, S.C.; Chi, X.; Schwartz, R.; Zaffran, S.; Niederreither, K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA 2008, 105, 2913–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wobus, A.M.; Kaomei, G.; Shan, J.; Wellner, M.C.; Rohwedel, J.; Ji, G.; Fleischmann, B.; Katus, H.A.; Hescheler, J.; Franz, W.M. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1997, 29, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Gassanov, N.; Er, F.; Zagidullin, N.; Jankowski, M.; Gutkowska, J.; Hoppe, U.C. Retinoid acid-induced effects on atrial and pacemaker cell differentiation and expression of cardiac ion channels. Differentiation 2008, 76, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Hochgreb, T.; Linhares, V.L.; Menezes, D.C.; Sampaio, A.C.; Yan, C.Y.; Cardoso, W.V.; Rosenthal, N.; Xavier-Neto, J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 2003, 130, 5363–5374. [Google Scholar] [CrossRef] [Green Version]
- Xavier-Neto, J.; Neville, C.M.; Shapiro, M.D.; Houghton, L.; Wang, G.F.; Nikovits, W., Jr.; Stockdale, F.E.; Rosenthal, N. A retinoic acid-inducible transgenic marker of sino-atrial development in the mouse heart. Development 1999, 126, 2677–2687. [Google Scholar]
- Niederreither, K.; Vermot, J.; Messaddeq, N.; Schuhbaur, B.; Chambon, P.; Dollé, P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 2001, 128, 1019–1031. [Google Scholar]
- Colbert, M.C.; Kirby, M.L.; Robbins, J. Endogenous retinoic acid signaling colocalizes with advanced expression of the adult smooth muscle myosin heavy chain isoform during development of the ductus arteriosus. Circ. Res. 1996, 78, 790–798. [Google Scholar] [CrossRef]
- Guadix, J.A.; Ruiz-Villalba, A.; Lettice, L.; Velecela, V.; Muñoz-Chápuli, R.; Hastie, N.D.; Pérez-Pomares, J.M.; Martínez-Estrada, O.M. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development 2011, 138, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Gruber, P.J.; Kubalak, S.W.; Pexieder, T.; Sucov, H.M.; Evans, R.M.; Chien, K.R. RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J. Clin. Investig. 1996, 98, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Pashmforoush, M.; Sucov, H.M. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev. Cell 2010, 18, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, E.; Sucov, H.M.; Kubalak, S.W.; Schmid-Schönbein, G.W.; DeLano, F.A.; Evans, R.M.; Ross, J., Jr.; Chien, K.R. Atrial-like phenotype is associated with embryonic ventricular failure in retinoid X receptor alpha -/- mice. Proc. Natl. Acad. Sci. USA 1995, 92, 7386–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucov, H.M.; Dyson, E.; Gumeringer, C.L.; Price, J.; Chien, K.R.; Evans, R.M. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994, 8, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.Y.; Luo, J.; Evans, R.M.; Giguere, V.; Sucov, H.M. Compartment-selective sensitivity of cardiovascular morphogenesis to combinations of retinoic acid receptor gene mutations. Circ. Res. 1997, 80, 757–764. [Google Scholar] [CrossRef]
- D’Aniello, E.; Rydeen, A.B.; Anderson, J.L.; Mandal, A.; Waxman, J.S. Depletion of retinoic acid receptors initiates a novel positive feedback mechanism that promotes teratogenic increases in retinoic acid. PLoS Genet. 2013, 9, e1003689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydeen, A.B.; Waxman, J.S. Cyp26 enzymes are required to balance the cardiac and vascular lineages within the anterior lateral plate mesoderm. Development 2014, 141, 1638–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydeen, A.B.; Waxman, J.S. Cyp26 Enzymes Facilitate Second Heart Field Progenitor Addition and Maintenance of Ventricular Integrity. PLoS Biol. 2016, 14, e2000504. [Google Scholar] [CrossRef]
- Rydeen, A.; Voisin, N.; D’Aniello, E.; Ravisankar, P.; Devignes, C.S.; Waxman, J.S. Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling. Dev. Biol. 2015, 405, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Marshall, H.; Nonchev, S.; Sham, M.H.; Muchamore, I.; Lumsden, A.; Krumlauf, R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature 1992, 360, 737–741. [Google Scholar] [CrossRef]
- Marshall, H.; Studer, M.; Pöpperl, H.; Aparicio, S.; Kuroiwa, A.; Brenner, S.; Krumlauf, R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature 1994, 370, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Roux, M.; Ryckebüsch, L.; Niederreither, K.; Dollé, P.; Moon, A.; Capecchi, M.; Zaffran, S. Hox genes define distinct progenitor sub-domains within the second heart field. Dev. Biol. 2011, 353, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waxman, J.S.; Keegan, B.R.; Roberts, R.W.; Poss, K.D.; Yelon, D. Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev. Cell 2008, 15, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Waxman, J.S.; Yelon, D. Increased Hox activity mimics the teratogenic effects of excess retinoic acid signaling. Dev. Dyn. 2009, 238, 1207–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostetskii, I.; Jiang, Y.; Kostetskaia, E.; Yuan, S.; Evans, T.; Zile, M. Retinoid signaling required for normal heart development regulates GATA-4 in a pathway distinct from cardiomyocyte differentiation. Dev. Biol. 1999, 206, 206–218. [Google Scholar] [CrossRef] [PubMed]
- De Bono, C.; Thellier, C.; Bertrand, N.; Sturny, R.; Jullian, E.; Cortes, C.; Stefanovic, S.; Zaffran, S.; Théveniau-Ruissy, M.; Kelly, R.G. T-box genes and retinoic acid signaling regulate the segregation of arterial and venous pole progenitor cells in the murine second heart field. Hum. Mol. Genet. 2018, 27, 3747–3760. [Google Scholar] [CrossRef] [PubMed]
- Matt, N.; Dupé, V.; Garnier, J.M.; Dennefeld, C.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005, 132, 4789–4800. [Google Scholar] [CrossRef] [Green Version]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
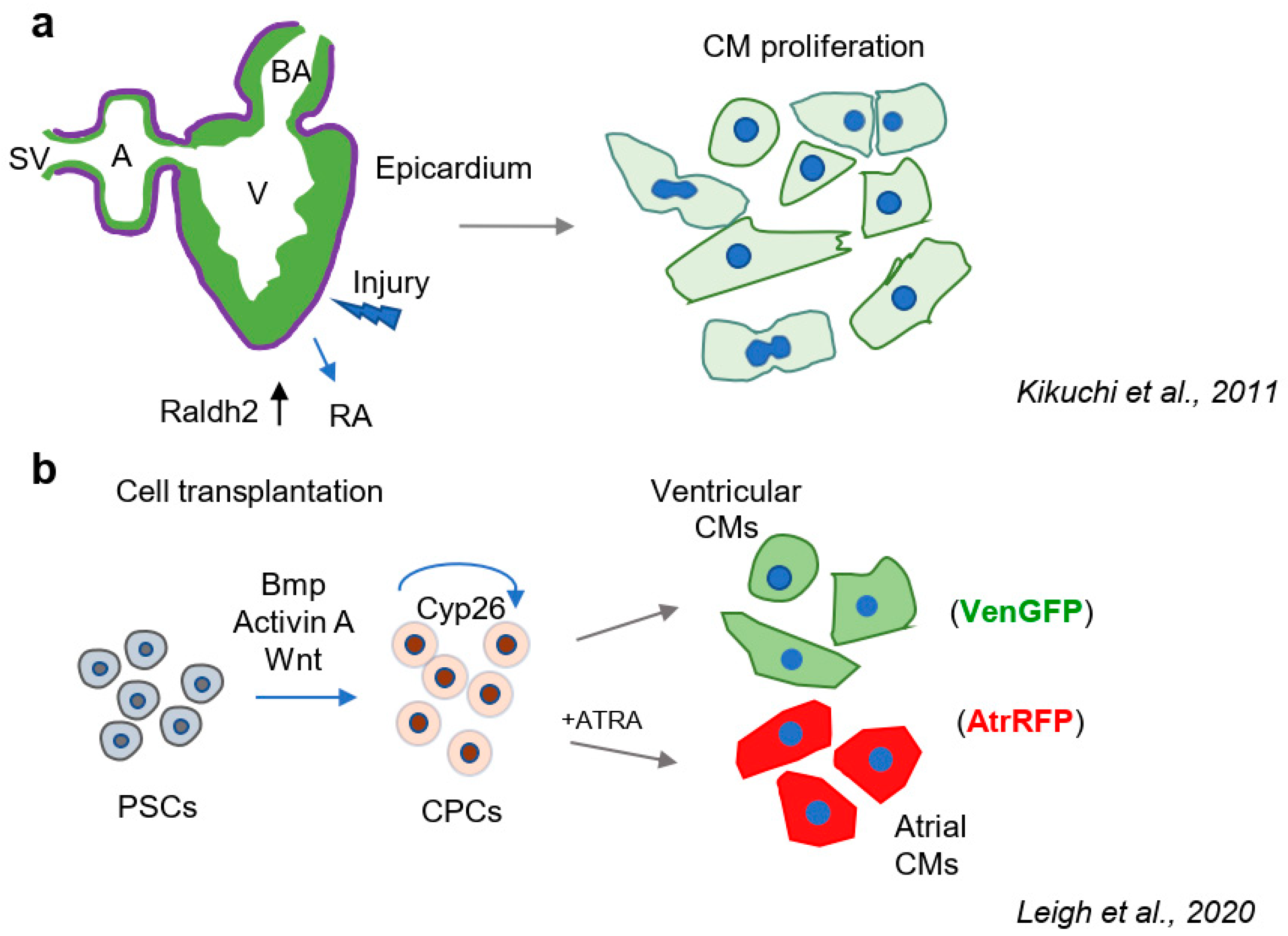
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 2006, 127, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Stuckmann, I.; Evans, S.; Lassar, A.B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev. Biol. 2003, 255, 334–349. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pomares, J.M.; Phelps, A.; Sedmerova, M.; Carmona, R.; González-Iriarte, M.; Muñoz-Chápuli, R.; Wessels, A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: A model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev. Biol. 2002, 247, 307–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brade, T.; Kumar, S.; Cunningham, T.J.; Chatzi, C.; Zhao, X.; Cavallero, S.; Li, P.; Sucov, H.M.; Ruiz-Lozano, P.; Duester, G. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development 2011, 138, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Jones-Villeneuve, E.M.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef]
- McBurney, M.W.; Jones-Villeneuve, E.M.; Edwards, M.K.; Anderson, P.J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 1982, 299, 165–167. [Google Scholar] [CrossRef]
- Strickland, S.; Mahdavi, V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell 1978, 15, 393–403. [Google Scholar] [CrossRef]
- Branco, A.F.; Pereira, S.P.; Gonzalez, S.; Gusev, O.; Rizvanov, A.A.; Oliveira, P.J. Gene Expression Profiling of H9c2 Myoblast Differentiation towards a Cardiac-Like Phenotype. PLoS ONE 2015, 10, e0129303. [Google Scholar] [CrossRef] [Green Version]
- Ménard, C.; Pupier, S.; Mornet, D.; Kitzmann, M.; Nargeot, J.; Lory, P. Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. J. Biol. Chem. 1999, 274, 29063–29070. [Google Scholar] [CrossRef] [Green Version]
- Leigh, R.S.; Ruskoaho, H.J.; Kaynak, B.L. A novel dual reporter embryonic stem cell line for toxicological assessment of teratogen-induced perturbation of anterior-posterior patterning of the heart. Arch. Toxicol. 2020, 94, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jiang, J.; Han, P.; Yuan, Q.; Zhang, J.; Zhang, X.; Xu, Y.; Cao, H.; Meng, Q.; Chen, L.; et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011, 21, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Protze, S.I.; Laksman, Z.; Backx, P.H.; Keller, G.M. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 2017, 21, 179–194. [Google Scholar] [CrossRef]
- Drowley, L.; McPheat, J.; Nordqvist, A.; Peel, S.; Karlsson, U.; Martinsson, S.; Müllers, E.; Dellsén, A.; Knight, S.; Barrett, I.; et al. Discovery of retinoic acid receptor agonists as proliferators of cardiac progenitor cells through a phenotypic screening approach. Stem Cells Transl. Med. 2020, 9, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Kurl, S. Low serum lycopene and β-carotene increase risk of acute myocardial infarction in men. Eur. J. Public Health 2012, 22, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Laukkanen, J.A.; Mäkikallio, T.H.; Ronkainen, K.; Kurl, S. Low β-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Min, K.B.; Min, J.Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1197–1203. [Google Scholar] [CrossRef]
- Olsen, T.; Vinknes, K.J.; Svingen, G.F.T.; Pedersen, E.R.; Tell, G.S.; Blomhoff, R.; Drevon, C.A.; Ueland, P.M.; Midttun, Ø.; Refsum, H.; et al. Cardiovascular disease risk associated with serum apolipoprotein B is modified by serum vitamin A. Atherosclerosis 2017, 265, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Saha, N.; Ng, T.B.; Tan, P.Y.; Wee, K.P. Vitamin A reserve of liver in health and coronary heart disease among ethnic groups in Singapore. Br. J. Nutr. 1988, 60, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Guleria, R.S.; Thomas, C.M.; Roth, A.; Gerilechaogetu, F.; Kumar, R.; Dostal, D.E.; Baker, K.M.; Pan, J. Loss of myocardial retinoic acid receptor α induces diastolic dysfunction by promoting intracellular oxidative stress and calcium mishandling in adult mice. J. Mol. Cell. Cardiol. 2016, 99, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Persaud, S.D.; Ogokeh, S.; Meyers, T.A.; Townsend, D.; Wei, L.N. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J. Endocrinol. 2018, 236, 151–165. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Zhou, L.; Lu, L.M.; Zhu, Y.C.; Yao, T. All-trans retinoic acid inhibited angiotensin II-induced increase in cell growth and collagen secretion of neonatal cardiac fibroblasts. Acta Pharmacol. Sin. 2006, 27, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, S.A.; Matsubara, L.S.; Matsubara, B.B.; Minicucci, M.F.; Azevedo, P.S.; Campana, A.O.; Zornoff, L.A. Retinoic acid supplementation attenuates ventricular remodeling after myocardial infarction in rats. J. Nutr. 2005, 135, 2326–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colbert, M.C.; Hall, D.G.; Kimball, T.R.; Witt, S.A.; Lorenz, J.N.; Kirby, M.L.; Hewett, T.E.; Klevitsky, R.; Robbins, J. Cardiac compartment-specific overexpression of a modified retinoic acid receptor produces dilated cardiomyopathy and congestive heart failure in transgenic mice. J. Clin. Investig. 1997, 100, 1958–1968. [Google Scholar] [CrossRef] [Green Version]
- Manolescu, D.C.; Jankowski, M.; Danalache, B.A.; Wang, D.; Broderick, T.L.; Chiasson, J.L.; Gutkowska, J. All-trans retinoic acid stimulates gene expression of the cardioprotective natriuretic peptide system and prevents fibrosis and apoptosis in cardiomyocytes of obese ob/ob mice. Appl. Physiol. Nutr. Metab. 2014, 39, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Lu, M.; Wang, H.; Tang, F. Retinoic acid attenuates cardiac injury induced by hyperglycemia in pre- and post-delivery mice. Can. J. Physiol. Pharmacol. 2020, 98, 6–14. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Azevedo, P.S.; Minicucci, M.E.; Rafacho, B.P.; Duarte, D.R.; Matsubara, L.S.; Matsubara, B.B.; Paiva, S.A.; Zornoff, L.A. Retinoic acid prevents ventricular remodelling induced by tobacco smoke exposure in rats. Acta Cardiol. 2011, 66, 3–7. [Google Scholar] [CrossRef]
- Subramanian, U.; Nagarajan, D. All-Trans Retinoic Acid supplementation prevents cardiac fibrosis and cytokines induced by Methylglyoxal. Glycoconj. J. 2017, 34, 255–265. [Google Scholar] [CrossRef]
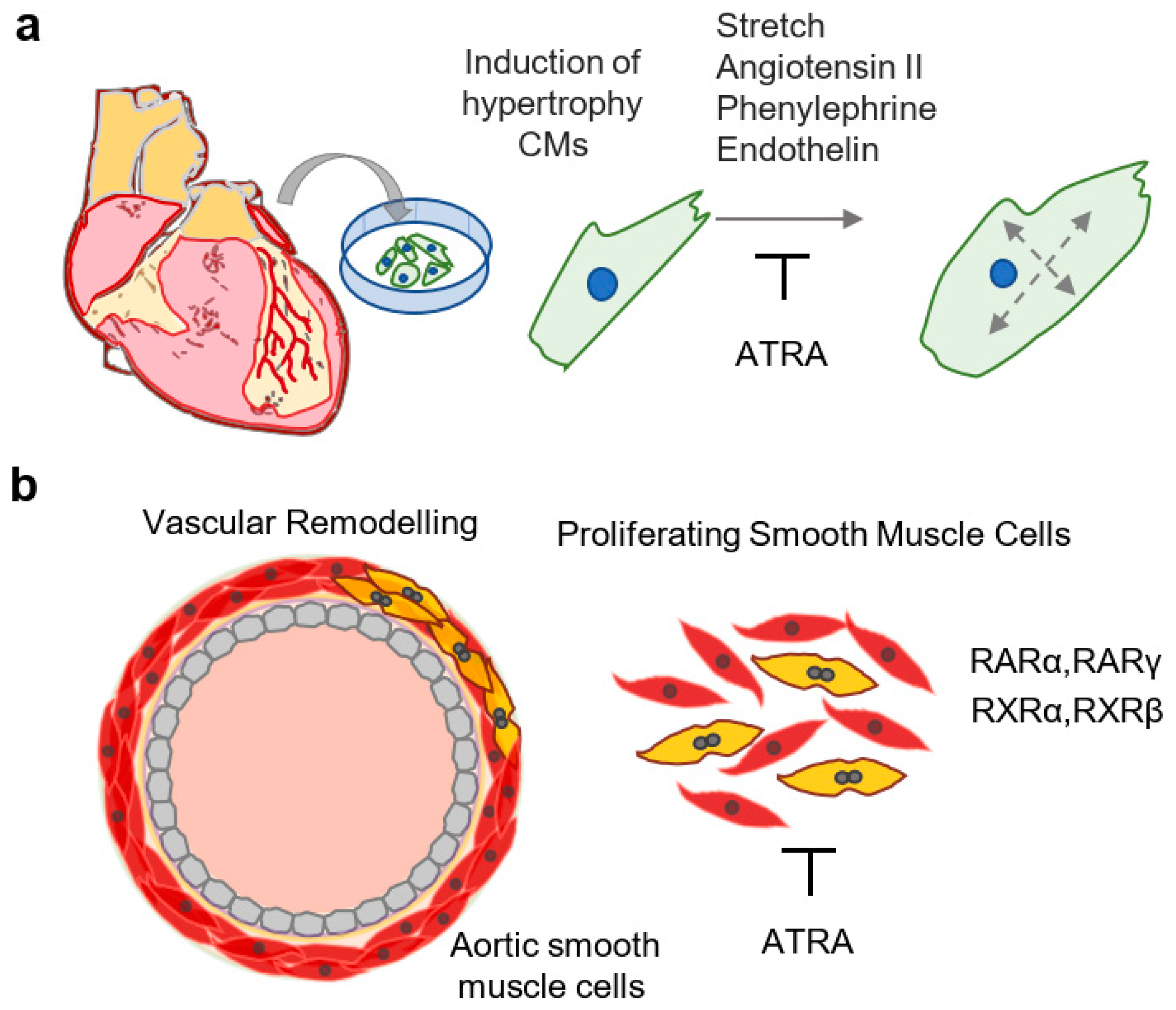
- Zhou, M.D.; Sucov, H.M.; Evans, R.M.; Chien, K.R. Retinoid-dependent pathways suppress myocardial cell hypertrophy. Proc. Natl. Acad. Sci. USA 1995, 92, 7391–7395. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Garami, M.; Cheng, T.; Gardner, D.G. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J. Clin. Investig. 1996, 97, 1577–1588. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.; Palm-Leis, A.; Scott, R.C., 3rd; Guleria, R.S.; Rachut, E.; Baker, K.M.; Pan, J. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H633–H644. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Nhieu, J.; Lin, Y.W.; Wei, L.N. All-trans retinoic acid attenuates isoproterenol-induced cardiac dysfunction through Crabp1 to dampen CaMKII activation. Eur. J. Pharmacol. 2019, 858, 172485. [Google Scholar] [CrossRef]
- Sultan, F.; Kaur, R.; Mir, A.H.; Maqbool, I.; Lonare, M.; Singh, D.; Rampal, S.; Dar, J.A. Rosuvastatin and retinoic acid may act as ‘pleiotropic agents’ against β-adrenergic agonist-induced acute myocardial injury through modulation of multiple signalling pathways. Chem. Biol. Interact. 2020, 318, 108970. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Leaf, A. Protective effects of All-trans-retinoic acid against cardiac arrhythmias induced by isoproterenol, lysophosphatidylcholine or ischemia and reperfusion. J. Cardiovasc. Pharmacol. 1995, 26, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.S.; Minicucci, M.F.; Chiuso-Minicucci, F.; Justulin, L.A., Jr.; Matsubara, L.S.; Matsubara, B.B.; Novelli, E.; Seiva, F.; Ebaid, G.; Campana, A.O.; et al. Ventricular remodeling induced by tissue vitamin A deficiency in rats. Cell. Physiol. Biochem. 2010, 26, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, M.F.; Azevedo, P.S.; Oliveira, S.A., Jr.; Martinez, P.F.; Chiuso-Minicucci, F.; Polegato, B.F.; Justulin, L.A., Jr.; Matsubara, L.S.; Matsubara, B.B.; Paiva, S.A.; et al. Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell. Physiol. Biochem. 2010, 26, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asson-Batres, M.A.; Ryzhov, S.; Tikhomirov, O.; Duarte, C.W.; Congdon, C.B.; Lessard, C.R.; McFarland, S.; Rochette-Egly, C.; Tran, T.L.; Galindo, C.L.; et al. Effects of vitamin A deficiency in the postnatal mouse heart: Role of hepatic retinoid stores. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1773–H1789. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, S.A.; Zornoff, L.A.; Okoshi, M.P.; Okoshi, K.; Matsubara, L.S.; Matsubara, B.B.; Cicogna, A.C.; Campana, A.O. Ventricular remodeling induced by retinoic acid supplementation in adult rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2242–H2246. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.A.C.; Gonçalves, A.F.; Dos Santos, P.P.; Rafacho, B.; Claro, R.F.T.; Minicucci, M.F.; Azevedo, P.S.; Polegato, B.F.; Zanati, S.G.; Fernandes, A.A.; et al. Cardiac Remodeling Induced by All-Trans Retinoic Acid is Detrimental in Normal Rats. Cell. Physiol. Biochem. 2017, 43, 1449–1459. [Google Scholar] [CrossRef]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Miano, J.M.; Topouzis, S.; Majesky, M.W.; Olson, E.N. Retinoid receptor expression and all-trans retinoic acid-mediated growth inhibition in vascular smooth muscle cells. Circulation 1996, 93, 1886–1895. [Google Scholar] [CrossRef]
- Neuville, P.; Yan, Z.; Gidlöf, A.; Pepper, M.S.; Hansson, G.K.; Gabbiani, G.; Sirsjö, A. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARalpha-dependent signaling pathway. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1430–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakala, R.; Benedict, C.R. RAR gamma agonists inhibit proliferation of vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2000, 35, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, C.; Sasaguri, T.; Komiyama, Y.; Takahashi, H. All-trans retinoic acid inhibits vascular smooth muscle cell proliferation targeting multiple genes for cyclins and cyclin-dependent kinases. Hypertens. Res. 2001, 24, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, A.; Francesconi, A.; Cocchia, D.; Corsini, A.; Spagnoli, L.G. Phenotypic heterogeneity influences apoptotic susceptibility to retinoic acid and cis-platinum of rat arterial smooth muscle cells in vitro: Implications for the evolution of experimental intimal thickening. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1118–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, H.; Haendeler, J.; Aebly, M.R.; Kelly, L.A.; Cholewa, B.C.; Koike, G.; Kwitek-Black, A.; Jacob, H.J.; Berk, B.C.; Miano, J.M. Retinoic acid-induced tissue transglutaminase and apoptosis in vascular smooth muscle cells. Circ. Res. 2000, 87, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Axel, D.I.; Frigge, A.; Dittmann, J.; Runge, H.; Spyridopoulos, I.; Riessen, R.; Viebahn, R.; Karsch, K.R. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Johst, U.; Betsch, A.; Wiskirchen, J.; Schöber, W.; Vonthein, R.; Rinkert, N.; Kehlbach, R.; Claussen, C.D.; Duda, S.H. All-trans and 9-cis retinoid acids inhibit proliferation, migration, and synthesis of extracellular matrix of human vascular smooth muscle cells by inducing differentiation in vitro. J. Cardiovasc. Pharmacol. 2003, 41, 526–535. [Google Scholar] [CrossRef]
- Wakino, S.; Kintscher, U.; Kim, S.; Jackson, S.; Yin, F.; Nagpal, S.; Chandraratna, R.A.; Hsueh, W.A.; Law, R.E. Retinoids inhibit proliferation of human coronary smooth muscle cells by modulating cell cycle regulators. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Gidlof, A.C.; Ocaya, P.; Olofsson, P.S.; Torma, H.; Sirsjo, A. Differences in retinol metabolism and proliferative response between neointimal and medial smooth muscle cells. J. Vasc. Res. 2006, 43, 392–398. [Google Scholar] [CrossRef]
- Bilbija, D.; Elmabsout, A.A.; Sagave, J.; Haugen, F.; Bastani, N.; Dahl, C.P.; Gullestad, L.; Sirsjö, A.; Blomhoff, R.; Valen, G. Expression of retinoic acid target genes in coronary artery disease. Int. J. Mol. Med. 2014, 33, 677–686. [Google Scholar] [CrossRef]
- Saito, A.; Sugawara, A.; Uruno, A.; Kudo, M.; Kagechika, H.; Sato, Y.; Owada, Y.; Kondo, H.; Sato, M.; Kurabayashi, M.; et al. All-trans retinoic acid induces in vitro angiogenesis via retinoic acid receptor: Possible involvement of paracrine effects of endogenous vascular endothelial growth factor signaling. Endocrinology 2007, 148, 1412–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.W.; Park, S.J.; Park, S.W.; Kim, J.J.; Hong, M.K.; Song, J.K. All-trans-retinoic acid attenuates neointima formation with acceleration of reendothelialization in balloon-injured rat aorta. J. Korean Med. Sci. 2000, 15, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M.; Kelly, L.A.; Artacho, C.A.; Nuckolls, T.A.; Piantedosi, R.; Blaner, W.S. All-Trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation 1998, 98, 1219–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; He, B.; Zheng, D.; Zhang, S.; Liu, J.; Zhu, S. All-trans retinoic acid reduces intimal thickening after balloon angioplasty in atherosclerotic rabbits. Chin. Med. J. 1999, 112, 121–123. [Google Scholar] [PubMed]
- Herdeg, C.; Oberhoff, M.; Baumbach, A.; Schroeder, S.; Leitritz, M.; Blattner, A.; Siegel-Axel, D.I.; Meisner, C.; Karsch, K.R. Effects of local all-trans-retinoic acid delivery on experimental atherosclerosis in the rabbit carotid artery. Cardiovasc. Res. 2003, 57, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Leville, C.D.; Dassow, M.S.; Seabrook, G.R.; Jean-Claude, J.M.; Towne, J.B.; Cambria, R.A. All-trans-retinoic acid decreases vein graft intimal hyperplasia and matrix metalloproteinase activity in vivo. J. Surg. Res. 2000, 90, 183–190. [Google Scholar] [CrossRef]
- Fujiu, K.; Manabe, I.; Ishihara, A.; Oishi, Y.; Iwata, H.; Nishimura, G.; Shindo, T.; Maemura, K.; Kagechika, H.; Shudo, K.; et al. Synthetic retinoid Am80 suppresses smooth muscle phenotypic modulation and in-stent neointima formation by inhibiting KLF5. Circ. Res. 2005, 97, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Lü, L.; Yao, T.; Zhu, Y.Z.; Huang, G.Y.; Cao, Y.X.; Zhu, Y.C. Chronic all-trans retinoic acid treatment prevents medial thickening of intramyocardial and intrarenal arteries in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1370–H1377. [Google Scholar] [CrossRef] [Green Version]
- Wiegman, P.J.; Barry, W.L.; McPherson, J.A.; McNamara, C.A.; Gimple, L.W.; Sanders, J.M.; Bishop, G.G.; Powers, E.R.; Ragosta, M.; Owens, G.K.; et al. All-trans-retinoic acid limits restenosis after balloon angioplasty in the focally atherosclerotic rabbit: A favorable effect on vessel remodeling. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Medhora, M.M. Retinoic acid upregulates beta(1)-integrin in vascular smooth muscle cells and alters adhesion to fibronectin. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H382–H387. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.; Wang, S.; Bailey, G.; Reichenbecher, V.; Wright, G.L. Effect of retinoic acid on contractile competence of vascular smooth muscle. Am. J. Physiol. 1996, 270, H1363–H1370. [Google Scholar] [CrossRef]
- Wright, G.L.; Wang, S.; Fultz, M.E.; Arif, I.; Matthews, K.; Chertow, B.S. Effect of vitamin A deficiency on cardiovascular function in the rat. Can. J. Physiol. Pharmacol. 2002, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Chen, J.; Nallamshetty, S.; Pham, T.; Goto, S.; Muehlschlegel, J.D.; Libby, P.; Aikawa, M.; Aikawa, E.; Plutzky, J. Retinoids Repress Human Cardiovascular Cell Calcification With Evidence for Distinct Selective Retinoid Modulator Effects. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Huk, D.J.; Hammond, H.L.; Kegechika, H.; Lincoln, J. Increased dietary intake of vitamin A promotes aortic valve calcification in vivo. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Krivospitskaya, O.; Elmabsout, A.A.; Sundman, E.; Söderström, L.A.; Ovchinnikova, O.; Gidlöf, A.C.; Scherbak, N.; Norata, G.D.; Samnegård, A.; Törmä, H.; et al. A CYP26B1 polymorphism enhances retinoic acid catabolism and may aggravate atherosclerosis. Mol. Med. 2012, 18, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Krum, H. Drug discovery for heart failure: A new era or the end of the pipeline? Nat. Rev. Drug Discov. 2007, 6, 127–139. [Google Scholar] [CrossRef]
- Chen, S.; Gardner, D.G. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J. Clin. Investig. 1998, 102, 653–662. [Google Scholar] [CrossRef]
- Kumar, P.; Garg, R.; Bolden, G.; Pandey, K.N. Interactive roles of Ets-1, Sp1, and acetylated histones in the retinoic acid-dependent activation of guanylyl cyclase/atrial natriuretic peptide receptor-A gene transcription. J. Biol. Chem. 2010, 285, 37521–37530. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Zhu, Y.C.; Yao, T. Effects of all-trans retinoic acid on angiotensin II-induced myocyte hypertrophy. J. Appl. Physiol. 2002, 92, 2162–2168. [Google Scholar] [CrossRef] [Green Version]
- Haxsen, V.; Adam-Stitah, S.; Ritz, E.; Wagner, J. Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ. Res. 2001, 88, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Maillet, M.; Watt, A.J.; Schwartz, R.J.; Aronow, B.J.; Duncan, S.A.; Molkentin, J.D. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ. Res. 2006, 98, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arceci, R.J.; King, A.A.; Simon, M.C.; Orkin, S.H.; Wilson, D.B. Mouse GATA-4: A retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 1993, 13, 2235–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molkentin, J.D.; Kalvakolanu, D.V.; Markham, B.E. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol. Cell. Biol. 1994, 14, 4947–4957. [Google Scholar] [CrossRef] [Green Version]
- Pikkarainen, S.; Tokola, H.; Majalahti-Palviainen, T.; Kerkela, R.; Hautala, N.; Bhalla, S.S.; Charron, F.; Nemer, M.; Vuolteenaho, O.; Ruskoaho, H. GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J. Biol. Chem. 2003, 278, 23807–23816. [Google Scholar] [CrossRef] [Green Version]
- Rysä, J.; Tenhunen, O.; Serpi, R.; Soini, Y.; Nemer, M.; Leskinen, H.; Ruskoaho, H. GATA-4 is an angiogenic survival factor of the infarcted heart. Circ. Heart Fail. 2010, 3, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruneau, B.G.; Nemer, G.; Schmitt, J.P.; Charron, F.; Robitaille, L.; Caron, S.; Conner, D.A.; Gessler, M.; Nemer, M.; Seidman, C.E.; et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 2001, 106, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Koshiba-Takeuchi, K.; Mori, A.D.; Kaynak, B.L.; Cebra-Thomas, J.; Sukonnik, T.; Georges, R.O.; Latham, S.; Beck, L.; Henkelman, R.M.; Black, B.L.; et al. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature 2009, 461, 95–98. [Google Scholar] [CrossRef]
- Luna-Zurita, L.; Stirnimann, C.U.; Glatt, S.; Kaynak, B.L.; Thomas, S.; Baudin, F.; Samee, M.A.; He, D.; Small, E.M.; Mileikovsky, M.; et al. Complex Interdependence Regulates Heterotypic Transcription Factor Distribution and Coordinates Cardiogenesis. Cell 2016, 164, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Mazurek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef] [Green Version]
- Palm-Leis, A.; Singh, U.S.; Herbelin, B.S.; Olsovsky, G.D.; Baker, K.M.; Pan, J. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J. Biol. Chem. 2004, 279, 54905–54917. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Deng, B.; Jiang, X.; Cai, M.; Liu, N.; Zhang, S.; Tan, Y.; Huang, G.; Jin, W.; Liu, B.; et al. All-Trans-Retinoic Acid Suppresses Neointimal Hyperplasia and Inhibits Vascular Smooth Muscle Cell Proliferation and Migration via Activation of AMPK Signaling Pathway. Front. Pharmacol. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kanai, H.; Arai, M.; Sekiguchi, K.; Uchiyama, T.; Nagai, R.; Kurabayashi, M. Retinoids induce the PAI-1 gene expression through tyrosine kinase-dependent pathways in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2002, 39, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Das, N.P. Effects of vitamin A and its analogs on nonenzymatic lipid peroxidation in rat brain mitochondria. J. Neurochem. 1989, 52, 585–588. [Google Scholar] [CrossRef]
- Halevy, O.; Sklan, D. Inhibition of arachidonic acid oxidation by beta-carotene, retinol and alpha-tocopherol. Biochim. Biophys. Acta 1987, 918, 304–307. [Google Scholar] [CrossRef]
- Samokyszyn, V.M.; Marnett, L.J. Inhibition of liver microsomal lipid peroxidation by 13-cis-retinoic acid. Free Radic. Biol. Med. 1990, 8, 491–496. [Google Scholar] [CrossRef]
- Vile, G.F.; Winterbourn, C.C. Inhibition of adriamycin-promoted microsomal lipid peroxidation by beta-carotene, alpha-tocopherol and retinol at high and low oxygen partial pressures. FEBS Lett. 1988, 238, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Tesoriere, L.; Ciaccio, M.; Valenza, M.; Bongiorno, A.; Maresi, E.; Albiero, R.; Livrea, M.A. Effect of vitamin A administration on resistance of rat heart against doxorubicin-induced cardiotoxicity and lethality. J. Pharmacol. Exp. Ther. 1994, 269, 430–436. [Google Scholar]
- Danelisen, I.; Palace, V.; Lou, H.; Singal, P.K. Maintenance of myocardial levels of vitamin A in heart failure due to adriamycin. J. Mol. Cell. Cardiol. 2002, 34, 789–795. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, J.; Zhao, X.; Yang, K.; Lu, L.; Zhang, F.; Shen, W.; Zhang, R. All-Trans Retinoic Acid Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Cardiomyocyte Apoptosis. PLoS ONE 2015, 10, e0133414. [Google Scholar] [CrossRef]
- Vega, V.A.; Anzulovich, A.C.; Varas, S.M.; Bonomi, M.R.; Giménez, M.S.; Oliveros, L.B. Effect of nutritional vitamin A deficiency on lipid metabolism in the rat heart: Its relation to PPAR gene expression. Nutrition 2009, 25, 828–838. [Google Scholar] [CrossRef]
- Cresci, S.; Clabby, M.L.; Kelly, D.P. Evidence for a novel cardiac-enriched retinoid X receptor partner. J. Biol. Chem. 1999, 274, 25668–25674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtamo, J.; Rapola, J.M.; Ripatti, S.; Heinonen, O.P.; Taylor, P.R.; Albanes, D.; Huttunen, J.K. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch. Intern. Med. 1998, 158, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Simioniuc, A.; Campan, M.; Lionetti, V.; Marinelli, M.; Aquaro, G.D.; Cavallini, C.; Valente, S.; Di Silvestre, D.; Cantoni, S.; Bernini, F.; et al. Placental stem cells pre-treated with a hyaluronan mixed ester of butyric and retinoic acid to cure infarcted pig hearts: A multimodal study. Cardiovasc. Res. 2011, 90, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Maioli, M.; Asara, Y.; Santoni, D.; Scarlata, I.; Cantoni, S.; Perbellini, A. Butyric and retinoic mixed ester of hyaluronan. A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells. J. Biol. Chem. 2004, 279, 23574–23579. [Google Scholar] [CrossRef] [Green Version]
- Ventura, C.; Cantoni, S.; Bianchi, F.; Lionetti, V.; Cavallini, C.; Scarlata, I.; Foroni, L.; Maioli, M.; Bonsi, L.; Alviano, F.; et al. Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J. Biol. Chem. 2007, 282, 14243–14252. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, V.; Cantoni, S.; Cavallini, C.; Bianchi, F.; Valente, S.; Frascari, I.; Olivi, E.; Aquaro, G.D.; Bonavita, F.; Scarlata, I.; et al. Hyaluronan mixed esters of butyric and retinoic acid affording myocardial survival and repair without stem cell transplantation. J. Biol. Chem. 2010, 285, 9949–9961. [Google Scholar] [CrossRef] [Green Version]
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early inhibition of endothelial retinoid uptake upon myocardial infarction restores cardiac function and prevents cell, tissue, and animal death. J. Mol. Cell. Cardiol. 2019, 126, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Messner, B.; Kern, J.; Wiedemann, D.; Schwaiger, S.; Türkcan, A.; Ploner, C.; Trockenbacher, A.; Aumayr, K.; Bonaros, N.; Laufer, G.; et al. 5-Methoxyleoligin, a lignan from Edelweiss, stimulates CYP26B1-dependent angiogenesis in vitro and induces arteriogenesis in infarcted rat hearts in vivo. PLoS ONE 2013, 8, e58342. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Abbreviation | Full Name | Abbreviation | Full Name |
|---|---|---|---|
| 9CRA | 9-cis retinoic acid | PSC | pluripotent stem cell |
| ATRA | all-trans retinoic acid | RA | retinoic acid |
| atrRFP | atrial RFP reporter | RAR | retinoic acid receptor |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II | RARE | retinoic acid response element |
| CHD | congenital heart disease | RBP | retinol binding protein |
| CPC | cardiac progenitor cell | RXR | retinoid x receptor |
| CRBP | cellular retinol binding protein | SMC | smooth muscle cell |
| CVD | cardiovascular disease | venGFP | ventricular GFP reporter |
| MI | myocardial infarction | VD3 | vitamin D3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leigh, R.S.; Kaynak, B.L. Vitamin A as a Transcriptional Regulator of Cardiovascular Disease. Hearts 2020, 1, 126-145. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts1020013
Leigh RS, Kaynak BL. Vitamin A as a Transcriptional Regulator of Cardiovascular Disease. Hearts. 2020; 1(2):126-145. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts1020013
Chicago/Turabian StyleLeigh, Robert S., and Bogac L. Kaynak. 2020. "Vitamin A as a Transcriptional Regulator of Cardiovascular Disease" Hearts 1, no. 2: 126-145. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts1020013





