Diagnosis, Prevention, Treatment and Surveillance of Anthracycline-Induced Cardiovascular Toxicity in Pediatric Cancer Survivors
Abstract
:1. Introduction
2. Mechanisms and Risk Factors for Anthracycline Cardiotoxicity
3. Diagnosis
3.1. ECG
3.2. Non-Invasive Cardiac Imaging
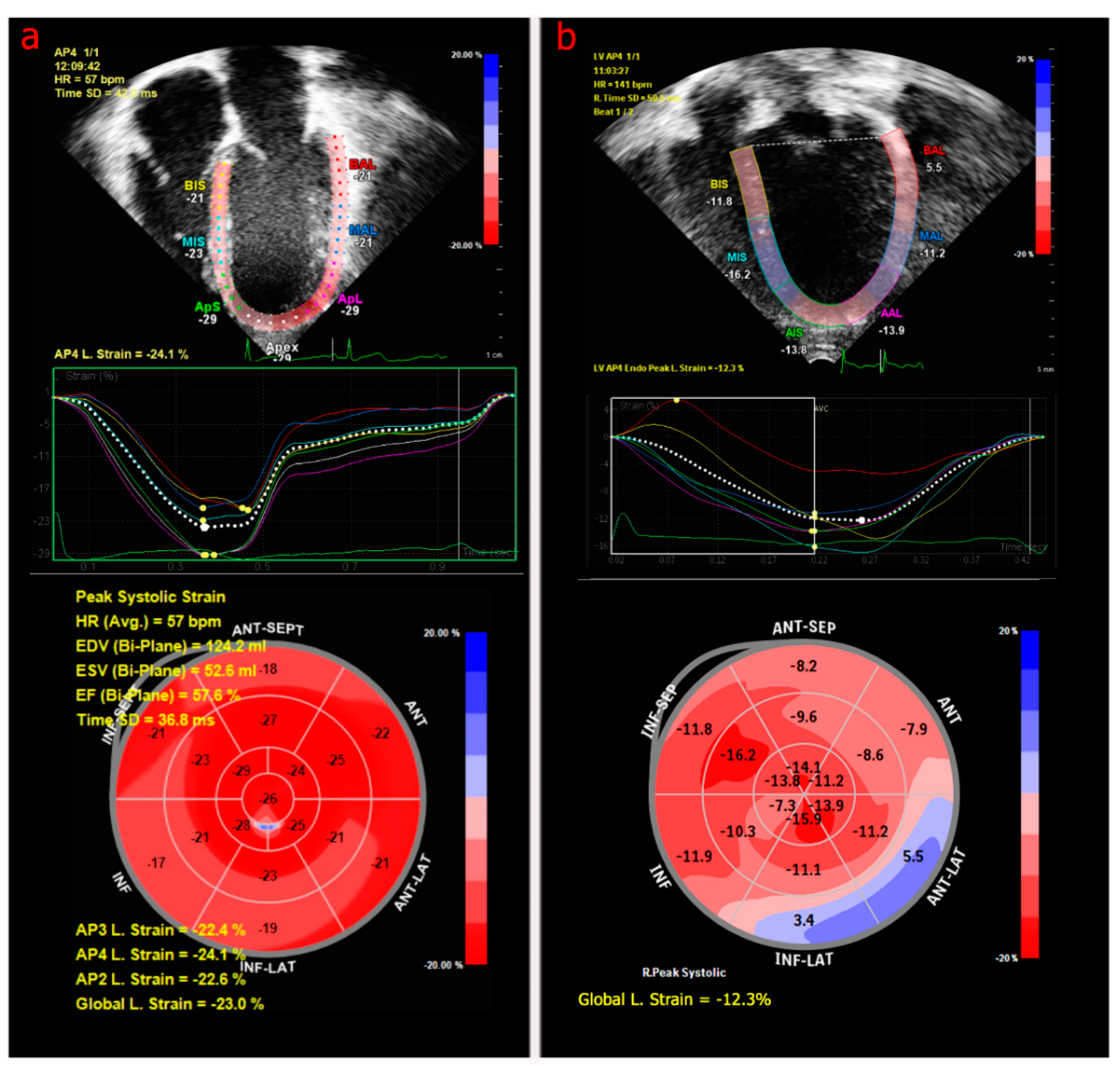
3.2.1. Echocardiography
3.2.2. Cardiac Magnetic Resonance Imaging
3.2.3. Serum Biomarkers
4. Prevention
5. Pediatric Heart Failure and Treatment Strategies
6. Screening Guidelines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes among Adults Treated for Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. JAMA J. Am. Med. Assoc. 2013, 309, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Neglia, J.P.; Leisenring, W.; Robison, L.L.; Mertens, A.C. Late Mortality Among 5-Year Survivors of Childhood Cancer: A Summary From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2328–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e603–e634. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-Term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, N.; Liu, Q.; Yeo, F.; Baassiri, M.; Ehrhardt, M.J.; Srivastava, D.K.; Metzger, M.L.; Krasin, M.J.; Ness, K.K.; Hudson, M.M.; et al. Cumulative Burden of Cardiovascular Morbidity in Paediatric, Adolescent, and Young Adult Survivors of Hodgkin’s Lymphoma: An Analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016, 17, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac Outcomes in a Cohort of Adult Survivors of Childhood and Adolescent Cancer: Retrospective Analysis of the Childhood Cancer Survivor Study Cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef] [Green Version]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Getz, K.D.; Sung, L.; Ky, B.; Gerbing, R.B.; Leger, K.J.; Leahy, A.B.; Sack, L.; Woods, W.G.; Alonzo, T.; Gamis, A.; et al. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2019, 37, 12–21. [Google Scholar] [CrossRef]
- Prout, M.N.; Richards, M.J.S.; Chung, K.J.; Joo, P.; Davis, H.L. Adriamycin Cardiotoxicity in Children. Case Reports, Literature Review, and Risk Factors. Cancer 1977, 39, 62–65. [Google Scholar] [CrossRef]
- Mertens, A.; Liu, Q.; Neglia, J.; Wasilewski, K.; Leisenring, W.; Armstrong, G.; Robison, L.; Yasui, Y. Cause-Specific Late Mortality Among 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Bottinor, W.J.; Friedman, D.L.; Ryan, T.D.; Wang, L.; Yu, C.; Borinstein, S.C.; Godown, J. Cardiovascular Disease and Asymptomatic Childhood Cancer Survivors: Current Clinical Practice. Cancer Med. 2020, 9, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Marwick, T.H. Role of Cardioprotective Therapy for Prevention of Cardiotoxicity with Chemotherapy: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2013, 49, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Alvarez, J.A.; Scully, R.E. Anthracycline Associated Cardiotoxicity in Survivors of Childhood Cancer. Heart 2008, 94, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creutzig, U.; Diekamp, S.; Zimmermann, M.; Reinhardt, D. Longitudinal Evaluation of Early and Late Anthracycline Cardiotoxicity in Children with AML. Pediatr. Blood Cancer 2007, 48, 651–662. [Google Scholar] [CrossRef]
- Gilladoga, A.C.; Manuel, C.; Tan, C.T.C.; Wollner, N.; Sternberg, S.S.; Murphy, M.L. The Cardiotoxicity of Adriamycin and Daunomycin in Children. Cancer 1976, 37 (Suppl. S2), 1070–1078. [Google Scholar] [CrossRef]
- Mancilla, T.R.; Iskra, B.; Aune, G.J. Doxorubicin-Induced Cardiomyopathy in Children. Compr. Physiol. 2019, 9, 905–931. [Google Scholar] [CrossRef]
- Adams, M.J.; Lipshultz, S.E. Pathophysiology of Anthracycline- and Radiation-Associated Cardiomyopathies: Implications for Screening and Prevention. Pediatr. Blood Cancer 2005, 44, 600–606. [Google Scholar] [CrossRef]
- Zhou, S.; Palmeira, C.M.; Wallace, K.B. Doxorubicin-Induced Persistent Oxidative Stress to Cardiac Myocytes. Toxicol. Lett. 2001, 121, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, K.H.; Kleppa, L.; Aronsen, J.M.; Eide, L.; Carlsen, H.; Haugen, Ø.P.; Sjaastad, I.; Klungland, A.; Rasmussen, L.J.; Attramadal, H.; et al. Impaired Dynamics and Function of Mitochondria Caused by MtDNA Toxicity Leads to Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H434–H449. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, B. Doxorubicin Induces Cardiotoxicity through Upregulation of Death Receptors Mediated Apoptosis in Cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef] [Green Version]
- Vejpongsa, P.; Yeh, E.T.H. Topoisomerase 2β: A Promising Molecular Target for Primary Prevention of Anthracycline-Induced Cardiotoxicity. Clin. Pharmacol. Ther. 2014, 95, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.L.; Kerrigan, J.E.; Lin, C.-P.; Azarova, A.M.; Tsai, Y.-C.; Ban, Y.; Liu, L.F. Topoisomerase IIβ–Mediated DNA Double-Strand Breaks: Implications in Doxorubicin Cardiotoxicity and Prevention by Dexrazoxane. Cancer Res. 2007, 67, 8839–8846. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, S.E.; Lipsitz, S.R.; Sallan, S.E.; Dalton, V.M.; Mone, S.M.; Gelber, R.D.; Colan, S.D. Chronic Progressive Cardiac Dysfunction Years After Doxorubicin Therapy for Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Ramil, J.M.; Rikka, S.; Kim, L.; Lee, Y.; Gude, N.A.; Thistlethwaite, P.A.; Sussman, M.A.; Gottlieb, R.A.; et al. Juvenile Exposure to Anthracyclines Impairs Cardiac Progenitor Cell Function and Vascularization Resulting in Greater Susceptibility to Stress-Induced Myocardial Injury in Adult Mice. Circulation 2010, 121, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.D.; Nagarajan, R.; Godown, J. Cardiovascular Toxicities in Pediatric Cancer Survivors. Cardiol. Clin. 2019, 37, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Mason, J.W.; Bristow, M.R.; Daniels, J.R. Anthracycline Cardiomyopathy Monitored by Morphologic Changes. Cancer Treat. Rep. 1978, 62, 865–872. [Google Scholar]
- Yeh, E.T.H.; Bickford, C.L. Cardiovascular Complications of Cancer Therapy. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, S.E.; Scully, R.E.; Stevenson, K.E.; Franco, V.I.; Neuberg, D.S.; Colan, S.D.; Silverman, L.B.; Moslehi, J.J.; Cheng, S.; Sallan, S.E. Hearts Too Small for Body Size after Doxorubicin for Childhood ALL: Grinch Syndrome. J. Clin. Oncol. 2014, 32, 10021. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer. N. Engl. J. Med. 1995, 332, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Meiners, B.; Shenoy, C.; Zordoky, B.N. Clinical and Preclinical Evidence of Sex-Related Differences in Anthracycline-Induced Cardiotoxicity. Biol. Sex Differ. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Aminkeng, F.; Bhavsar, A.P.; Visscher, H.; Rassekh, S.R.; Li, Y.; Lee, J.W.; Brunham, L.R.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. A Coding Variant in RARG Confers Susceptibility to Anthracycline-Induced Cardiotoxicity in Childhood Cancer. Nat. Genet. 2015, 47, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Ross, C.J.D.; Rassekh, S.R.; Barhdadi, A.; Dubé, M.-P.; Al-Saloos, H.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children. J. Clin. Oncol. 2011, 30, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Ross, C.J.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; Van Dalen, E.C.; Kremer, L.C.; Van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; et al. Validation of Variants in SLC28A3 and UGT1A6 as Genetic Markers Predictive of Anthracycline-Induced Cardiotoxicity in Children. Pediatr. Blood Cancer 2013, 60, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Visscher, H.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; Carleton, B.C.; et al. Genetic Variants in SLC22A17 and SLC22A7 Are Associated with Anthracycline-Induced Cardiotoxicity in Children. Pharmacogenomics 2015, 16, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Dengel, D.R.; Kelly, A.S.; Zhang, L.; Hodges, J.S.; Baker, K.S.; Steinberger, J. Signs of Early Sub-Clinical Atherosclerosis in Childhood Cancer Survivors. Pediatr. Blood Cancer 2014, 61, 532–537. [Google Scholar] [CrossRef]
- Dengel, D.; Ness, K.; Glasser, S.; Williamson, E.; Baker, K.; Gurney, J. Endothelial Function in Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2008, 30, 20–25. [Google Scholar] [CrossRef]
- Wong, F.L.; Bhatia, S.; Landier, W.; Francisco, L.; Leisenring, W.; Hudson, M.M.; Armstrong, G.T.; Mertens, A.; Stovall, M.; Robison, L.L.; et al. Efficacy and Cost-Effectiveness of the Children’s Oncology Group Long-Term Follow-Up Screening Guidelines for Childhood Cancer Survivors at Risk of Treatment-Related Heart Failure. Ann. Intern. Med. 2014, 160, 672–683. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Tao, J.; Zhai, M.; Li, C.; Zhou, N.; Lv, J.; Wang, L.; Lin, L.; Bai, R. Anticancer Drugs-Related QTc Prolongation, Torsade de Pointes and Sudden Death: Current Evidence and Future Research Perspectives. Oncotarget 2018, 9, 25738–25749. [Google Scholar] [CrossRef] [Green Version]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; Tissing, W.J.E.; Shankar, S.; Sieswerda, E.; et al. Recommendations for Cardiomyopathy Surveillance for Survivors of Childhood Cancer: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, K.; Levitt, G.; Bull, C.; Chessells, J.; Sullivan, I. Anthracycline Dose in Childhood Acute Lymphoblastic Leukemia: Issues of Early Survival versus Late Cardiotoxicity. J. Clin. Oncol. 1997, 15, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic Implications of Global LV Dysfunction: A Systematic Review and Meta-Analysis of Global Longitudinal Strain and Ejection Fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Joshi, V.M.; Ness, K.K.; Marwick, T.H.; Zhang, N.; Srivastava, D.; Griffin, B.P.; Grimm, R.A.; Thomas, J.; Phelan, D.; et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 2015, 65, 2511–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, G.T.; Plana, J.C.; Zhang, N.; Srivastava, D.; Green, D.M.; Ness, K.K.; Donovan, F.D.; Metzger, M.L.; Arevalo, A.; Durand, J.-B.; et al. Screening Adult Survivors of Childhood Cancer for Cardiomyopathy: Comparison of Echocardiography and Cardiac Magnetic Resonance Imaging. J. Clin. Oncol. 2012, 30, 2876–2884. [Google Scholar] [CrossRef]
- Border, W.L.; Sachdeva, R.; Stratton, K.L.; Armenian, S.H.; Bhat, A.; Cox, D.E.; Leger, K.J.; Leisenring, W.M.; Meacham, L.R.; Sadak, K.T.; et al. Longitudinal Changes in Echocardiographic Parameters of Cardiac Function in Pediatric Cancer Survivors. JACC CardioOncology 2020, 2, 26–37. [Google Scholar] [CrossRef]
- Venturelli, F.; Masetti, R.; Fabi, M.; Rondelli, R.; Martoni, A.; Prete, A.; Bonvicini, M.; Pession, A. Tissue Doppler Imaging for Anthracycline Cardiotoxicity Monitoring in Pediatric Patients with Cancer. Cardio-Oncology 2018, 4. [Google Scholar] [CrossRef]
- Bijnens, B.; Cikes, M.; Butakoff, C.; Sitges, M.; Crispi, F. Myocardial Motion and Deformation: What Does It Tell Us and How Does It Relate to Function? Fetal Diagn. Ther. 2012, 32, 5–16. [Google Scholar] [CrossRef]
- Abraham Theodore, P.; Dimaano Veronica, L.; Hsin-Yueh, L. Role of Tissue Doppler and Strain Echocardiography in Current Clinical Practice. Circulation 2007, 116, 2597–2609. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sheu, R.; Zimmerman, N.M.; Alfirevic, A.; Sale, S.; Gillinov, A.M.; Duncan, A.E. A Comparison of Global Longitudinal, Circumferential, and Radial Strain to Predict Outcomes after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1315–1322. [Google Scholar] [CrossRef]
- Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients during and after Cancer Chemotherapy: A Systematic Review; Centre for Reviews and Dissemination: York, UK, 2014.
- Bottinor, W.; Godown, J.; Coburn, G.; Soslow, J.; Borinstein, S.C. Implementing Strain Imaging to Identify Early Childhood Cancer Survivors at Risk for Cardiovascular Disease. J. Clin. Oncol. 2019, 37 (Suppl. S15), e23070. [Google Scholar] [CrossRef]
- Schuster, A.; Hor, K.N.; Kowallick, J.T.; Beerbaum, P.; Kutty, S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking. Circ. Cardiovasc. Imaging 2016, 9, e004077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved Detection of Myocardial Involvement in Acute Inflammatory Cardiomyopathies Using T2 Mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riesenkampff, E.; Messroghli, D.R.; Redington, A.N.; Grosse-Wortmann, L. Myocardial T1 Mapping in Pediatric and Congenital Heart Disease. Circ. Cardiovasc. Imaging 2015, 8, e002504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etesami, M.; Gilkeson, R.C.; Rajiah, P. Utility of Late Gadolinium Enhancement in Pediatric Cardiac MRI. Pediatr. Radiol. 2016, 46, 1096–1113. [Google Scholar] [CrossRef]
- Tham, E.B.; Haykowsky, M.J.; Chow, K.; Spavor, M.; Kaneko, S.; Khoo, N.S.; Pagano, J.J.; Mackie, A.S.; Thompson, R.B. Diffuse Myocardial Fibrosis by T1-Mapping in Children with Subclinical Anthracycline Cardiotoxicity: Relationship to Exercise Capacity, Cumulative Dose and Remodeling. J. Cardiovasc. Magn. Reson. 2013, 15, 48. [Google Scholar] [CrossRef] [Green Version]
- Toro-Salazar, O.H.; Gillan, E.; O’Loughlin, M.T.; Burke, G.S.; Ferranti, J.; Stainsby, J.; Liang, B.; Mazur, W.; Raman, S.V.; Hor, K.N. Occult Cardiotoxicity in Childhood Cancer Survivors Exposed to Anthracycline Therapy. Circ. Cardiovasc. Imaging 2013, 6, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Long, T.M.; Marsh, C.E.; Dembo, L.G.; Watson, P.; Wallman, K.E.; Walwyn, T.S.; Choong, C.S.; Naylor, L.H. Early Markers of Cardiovascular Injury in Childhood Leukaemia Survivors Treated with Anthracycline Chemotherapy. Cardio-Oncology 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Mokshagundam, D.; Olivieri, L.J.; McCarter, R.; Kim, A.; Sable, C.A.; Spurney, C.F.; Dham, N. Cardiac Changes in Pediatric Cancer Survivors. J. Investig. Med. 2020, 68, 1364–1369. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Miller, T.L.; Scully, R.E.; Lipsitz, S.R.; Rifai, N.; Silverman, L.B.; Colan, S.D.; Neuberg, D.S.; Dahlberg, S.E.; Henkel, J.M.; et al. Changes in Cardiac Biomarkers during Doxorubicin Treatment of Pediatric Patients with High-Risk Acute Lymphoblastic Leukemia: Associations with Long-Term Echocardiographic Outcomes. J. Clin. Oncol. 2012, 30, 1042–1049. [Google Scholar] [CrossRef]
- Wieland, A.; Kerbl, R.; Berghold, A.; Schwinger, W.; Mann, G.; Urban, C. C-Reactive Protein (CRP) as Tumor Marker in Pediatric and Adolescent Patients with Hodgkin Disease. Med. Pediatr. Oncol. 2003, 41, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, H.; Azanan, M.S.; Ghafar, S.S.A.; Oh, L.; Lau, K.H.; Thirunavakarasu, T.; Sedan, A.; Ibrahim, K.; Chan, A.; Chin, T.F.; et al. Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia Show Evidence of Chronic Inflammation and Cellular Aging. Cancer 2017, 123, 4207–4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshultz, S.E.; Nader, R.; Sallan, S.E.; Lipsitz, S.R.; Dalton, V.; Sacks, D.B.; Ottlinger, M.E. Predictive Value of Cardiac Troponin T in Pediatric Patients at Risk for Myocardial Injury. Circulation 1997, 96, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Hausner, E.; Herman, E.; Holt, G.D.; MacGregor, J.T.; Metz, A.L.; Murphy, E.; Rosenblum, I.Y.; Sistare, F.D.; York, M.J. Serum Troponins as Biomarkers of Drug-Induced Cardiac Toxicity. Toxicol. Pathol. 2004, 32, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Tricca, A.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Cipolla, C.M.; Fiorentini, C. Left Ventricular Dysfunction Predicted by Early Troponin I Release after High-Dose Chemotherapy. J. Am. Coll. Cardiol. 2000, 36, 517–522. [Google Scholar] [CrossRef]
- Raluca Maniu, D.; Blag, C.; Popa, G.; Bota, M.; Vlad, C.; Cainap, C.; Balacescu, O.; Pop, L.; Cainap, S.S. The Role of Biomarkers and Echocardiography in the Evaluation of Cardiotoxicity Risk in Children Treated for Leukemia. J. BUON 2018, 23, 122–131. [Google Scholar]
- Voleti, S.; Olivieri, L.; Hamann, K.; Gordish-Dressman, H.; Spurney, C. Troponin I Levels Correlate with Cardiac MR LGE and Native T1 Values in Duchenne Muscular Dystrophy Cardiomyopathy and Identify Early Disease Progression. Pediatr. Cardiol. 2020, 41, 1173–1179. [Google Scholar] [CrossRef]
- Zidan, A.; Sherief, L.M.; El-sheikh, A.; Saleh, S.H.; Shahbah, D.A.; Kamal, N.M.; Sherbiny, H.S.; Ahmad, H. NT-ProBNP as Early Marker of Subclinical Late Cardiotoxicity after Doxorubicin Therapy and Mediastinal Irradiation in Childhood Cancer Survivors. Dis. Mark. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.; Mincu, R.I.; Mrotzek, S.M.; Korste, S.; Neudorf, U.; Rassaf, T.; Totzeck, M. Cardiac Biomarkers for the Detection of Cardiotoxicity in Childhood Cancer—A Meta-analysis. ESC Heart Fail. 2020, 7, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Sherief, L.; Kamal, A.; Khalek, E.; Kamal, N.; Soliman, A.; Esh, A. Biomarkers and Early Detection of Late Onset Anthracycline-Induced Cardiotoxicity in Children. Hematol. Amst. Neth. 2012, 17, 151–156. [Google Scholar] [CrossRef]
- Slieker, M.G.; Fackoury, C.; Slorach, C.; Hui, W.; Friedberg, M.K.; Fan, C.-P.S.; Manlhiot, C.; Dillenburg, R.; Kantor, P.; Mital, S.; et al. Echocardiographic Assessment of Cardiac Function in Pediatric Survivors of Anthracycline-Treated Childhood Cancer. Circ. Cardiovasc. Imaging 2019, 12, e008869. [Google Scholar] [CrossRef] [PubMed]
- Kopp, L.M.; Womer, R.B.; Schwartz, C.L.; Ebb, D.H.; Franco, V.I.; Hall, D.; Barkauskas, D.A.; Krailo, M.D.; Grier, H.E.; Meyers, P.A.; et al. Effects of Dexrazoxane on Doxorubicin-Related Cardiotoxicity and Second Malignant Neoplasms in Children with Osteosarcoma: A Report from the Children’s Oncology Group. Cardio-Oncology 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asselin, B.L.; Devidas, M.; Chen, L.; Franco, V.I.; Pullen, J.; Borowitz, M.J.; Hutchison, R.E.; Ravindranath, Y.; Armenian, S.H.; Camitta, B.M.; et al. Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J. Clin. Oncol. 2016, 34, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshultz, S.E.; Scully, R.E.; Lipsitz, S.R.; Sallan, S.E.; Silverman, L.B.; Miller, T.L.; Barry, E.V.; Asselin, B.L.; Athale, U.; Clavell, L.A.; et al. Assessment of Dexrazoxane as a Cardioprotectant in Doxorubicin-Treated Children with High-Risk Acute Lymphoblastic Leukaemia: Long-Term Follow-up of a Prospective, Randomised, Multicentre Trial. Lancet Oncol. 2010, 11, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Schloemer, N.J.; Brickler, M.; Hoffmann, R.; Pan, A.; Simpson, P.; McFadden, V.; Block, J.; Tower, R.L.; Burke, M.J. Administration of Dexrazoxane Improves Cardiac Indices in Children and Young Adults with Acute Myeloid Leukemia (AML) While Maintaining Survival Outcomes. J. Pediatr. Hematol. Oncol. 2017, 39, e254–e258. [Google Scholar] [CrossRef]
- Getz, K.D.; Sung, L.; Alonzo, T.A.; Leger, K.J.; Gerbing, R.B.; Pollard, J.A.; Cooper, T.; Kolb, E.A.; Gamis, A.S.; Ky, B.; et al. Effect of Dexrazoxane on Left Ventricular Systolic Function and Treatment Outcomes in Patients With Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2398–2406. [Google Scholar] [CrossRef]
- Reichardt, P.; Tabone, M.-D.; Mora, J.; Morland, B.; Jones, R.L. Risk–Benefit of Dexrazoxane for Preventing Anthracycline-Related Cardiotoxicity: Re-Evaluating the European Labeling. Future Oncol. 2018, 14, 2663–2676. [Google Scholar] [CrossRef]
- Chicco, A.J.; Hydock, D.S.; Schneider, C.M.; Hayward, R. Low-Intensity Exercise Training during Doxorubicin Treatment Protects against Cardiotoxicity. J. Appl. Physiol. 2006, 100, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Marques-Aleixo, I.; Santos-Alves, E.; Mariani, D.; Rizo-Roca, D.; Padrão, A.I.; Rocha-Rodrigues, S.; Viscor, G.; Torrella, J.R.; Ferreira, R.; Oliveira, P.J.; et al. Physical Exercise Prior and during Treatment Reduces Sub-Chronic Doxorubicin-Induced Mitochondrial Toxicity and Oxidative Stress. Mitochondrion 2015, 20, 22–33. [Google Scholar] [CrossRef]
- Bourdon, A.; Grandy, S.A.; Keats, M.R. Aerobic Exercise and Cardiopulmonary Fitness in Childhood Cancer Survivors Treated with a Cardiotoxic Agent: A Meta-Analysis. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 2113–2123. [Google Scholar] [CrossRef]
- van der Pal, H.J.; van Dalen, E.C.; van Delden, E.; van Dijk, I.W.; Kok, W.E.; Geskus, R.B.; Sieswerda, E.; Oldenburger, F.; Koning, C.C.; van Leeuwen, F.E.; et al. High Risk of Symptomatic Cardiac Events in Childhood Cancer Survivors. J. Clin. Oncol. 2012, 30, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1- and Beta 2-Adrenergic-Receptor Subpopulations in Nonfailing and Failing Human Ventricular Myocardium: Coupling of Both Receptor Subtypes to Muscle Contraction and Selective Beta 1-Receptor down-Regulation in Heart Failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, J.D.; Bristow, M.R. Altered Beta-Adrenergic Receptor Gene Regulation and Signaling in Chronic Heart Failure. J. Mol. Cell. Cardiol. 2001, 33, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Abdalla, A.; Osman, M.; Haykal, T.; Chahine, A.; Ahmed, S.; Osman, K.; Hassan, M.; Bachuwa, G.; Bhatt, D.L. Meta-Analysis of Carvedilol for the Prevention of Anthracycline-Induced Cardiotoxicity. Am. J. Cardiol. 2018, 122, 1959–1964. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Tolba, O.A.; El-Shanshory, M.R.; El-Hawary, E.E. Protective Effect of Carvedilol on Adriamycin-Induced Left Ventricular Dysfunction in Children with Acute Lymphoblastic Leukemia. J. Card. Fail. 2012, 18, 607–613. [Google Scholar] [CrossRef]
- Momma, K. ACE Inhibitors in Pediatric Patients with Heart Failure. Paediatr. Drugs 2006, 8, 55–69. [Google Scholar] [CrossRef]
- Hiona, A.; Lee, A.S.; Nagendran, J.; Xie, X.; Connolly, A.J.; Robbins, R.C.; Wu, J.C. Pretreatment with Angiotensin-Converting Enzyme Inhibitor Improves Doxorubicin-Induced Cardiomyopathy via Preservation of Mitochondrial Function. J. Thorac. Cardiovasc. Surg. 2011, 142, 396–403.e3. [Google Scholar] [CrossRef] [Green Version]
- Duboc, D.; Meune, C.; Pierre, B.; Wahbi, K.; Eymard, B.; Toutain, A.; Berard, C.; Vaksmann, G.; Weber, S.; Bécane, H.-M. Perindopril Preventive Treatment on Mortality in Duchenne Muscular Dystrophy: 10 Years’ Follow-Up. Am. Heart J. 2007, 154, 596–602. [Google Scholar] [CrossRef]
- Silber, J.H.; Cnaan, A.; Clark, B.J.; Paridon, S.M.; Chin, A.J.; Rychik, J.; Hogarty, A.N.; Cohen, M.I.; Barber, G.; Rutkowski, M.; et al. Enalapril to Prevent Cardiac Function Decline in Long-Term Survivors of Pediatric Cancer Exposed to Anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef]
- Shaddy, R.; Canter, C.; Halnon, N.; Kochilas, L.; Rossano, J.; Bonnet, D.; Bush, C.; Zhao, Z.; Kantor, P.; Burch, M.; et al. Design for the Sacubitril/Valsartan (LCZ696) Compared with Enalapril Study of Pediatric Patients with Heart Failure Due to Systemic Left Ventricle Systolic Dysfunction (PANORAMA-HF Study). Am. Heart J. 2017, 193, 23–34. [Google Scholar] [CrossRef]
- Shugh, S.B.; Ryan, T.D. Heart Transplantation in Survivors of Childhood Cancer. Transl. Pediatr. 2019, 8, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Katzenstein, H.M. Heart Transplantation for Anthracycline Cardiomyopathy: Pump up the Volume. Pediatr. Transplant. 2017, 21. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.J.; Pahl, E.; Rusconi, P.G.; Boyle, G.J.; Parent, J.J.; Twist, C.J.; Kirklin, J.K.; Pruitt, E.; Bernstein, D. Cancer Recurrence and Mortality after Pediatric Heart Transplantation for Anthracycline Cardiomyopathy: A Report from the Pediatric Heart Transplant Study (PHTS) Group. Pediatr. Transplant. 2017, 21. [Google Scholar] [CrossRef] [PubMed]
- Madden, N.A.; Deng, C.; Fitch, T.; Effinger, K.E.; Zhang, C.; Goyal, S.; Tian, S.; Batcha, M.M.; Parashar, S.; Eaton, B.R.; et al. Adherence to Children’s Oncology Group (COG) long-term follow-up guidelines for echocardiogram screening in young adult survivors of childhood cancer. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Camilli, M.; Del Buono, M.G.; Crea, F.; Minotti, G. Acute Heart Failure 29 Years after Treatment for Childhood Cancer. JACC CardioOncol. 2020. [Google Scholar] [CrossRef]

| Recommendations | NCCN GUIDELINES Version 2.2020 Survivorship: Anthracycline-Induced Cardiac Toxicity | NCCN 1.2021 Adolescent and Young Adult | Children’s Oncology Group Long-Term Follow-up Guidelines Version 5.0, 2018 | The Dutch Childhood Oncology Group 2012 | International Guideline Harmonization Group for Late Effects of Childhood Cancer |
|---|---|---|---|---|---|
| Targeted cardiac history and physical exam | Yes | Yes | Recommended yearly | Not addressed | Yes—at all routine follow-up visits |
| Heart failure risk stratification | Yes—by history and screening echocardiogram | Yes—by radiation and anthracycline dosing | Not addressed | Not addressed | Yes—by radiation and anthracycline dosing |
| Timing of 2D screening echocardiogram | 1 year after completion of anthracycline therapy if high cumulative dose (>/= 250 mg/m2) or low dose with one heart failure risk factor * | Every 2–5 years depending on anthracycline dose or radiation exposure doses | Every 2–5 years depending on anthracycline dose or radiation exposure doses | Measuring LV systolic function with FS and or EF every 2–5 years depending on anthracycline dose or radiation exposure doses | Detailed 2D echocardiography—should not be limited to ventricular function alone—consider diastolic function |
| ECG | Consider based on individual risk | Baseline ECG recommended for radiation exposure >15 Gy | Baseline at entry into long term follow up | Not addressed | Not addressed |
| Advanced Imaging | Not addressed | Not addressed | Consider CMR if echocardiographic images are suboptimal | Radionuclide angiography if echocardiographic images are suboptimal | Radionuclide angiography or CMR in individuals for whom echocardiography is not technically feasible/optimal |
| Biomarkers | Consider use of biomarkers in high risk patients without evidence of structural disease by echo | Not addressed | Not addressed | Not recommended | Consider if symptomatic with preserved function or borderline function on primary surveillance |
| Timing of referral to cardiology | After identification of structural heart disease, even if asymptomatic. Management deferred to cardiologist | Consider 5–10 years after radiation doses >/= 35 Gy | Patients with subclinical abnormalities on screening evaluation, LV dysfunction, dysrhythmia or prolonged QTc interval; specific recommendations made for high risk female patients planning pregnancy | Consult a cardiologist when cardiac function is borderline abnormal (FS 25–29%, EF 45–49%) andreferral to a cardiologist when cardiac function is clearlyabnormal (FS < 25%, EF < 45% | Recommended when survivors develop cardiomyopathy |
| Preventative lifestyle and screening for modifiable risk factors | Yes—involve PCP | Optimize cardiovascular risk factors including blood pressure, blood glucose and lipid profile | Yes—encourage heart healthy diet, maintaining healthy weight and blood pressure | Not addressed | Yes |
| Participation in exercise | Recommended unless physically unable—assess readiness and current levels of fitness. | Not addressed | Recommended. If abnormal function or high-risk survivor, cardiology consult recommended to define limits | Not addressed | Recommended. If abnormal function or high risk survivor, cardiology consult recommended to define limits |
| Preventative medications | Not addressed | Not addressed | Not addressed | In a research setting only | Not addressed |
| Link to recommendations | https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf | https://www.nccn.org/professionals/physician_gls/pdf/aya.pdf | http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf | https://www.annalsofoncology.org/action/showPdf?pii=S0923-7534%2819%2938105-0 | https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC4485458/ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curren, V.; Dham, N.; Spurney, C. Diagnosis, Prevention, Treatment and Surveillance of Anthracycline-Induced Cardiovascular Toxicity in Pediatric Cancer Survivors. Hearts 2021, 2, 45-60. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010005
Curren V, Dham N, Spurney C. Diagnosis, Prevention, Treatment and Surveillance of Anthracycline-Induced Cardiovascular Toxicity in Pediatric Cancer Survivors. Hearts. 2021; 2(1):45-60. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010005
Chicago/Turabian StyleCurren, Valerie, Niti Dham, and Christopher Spurney. 2021. "Diagnosis, Prevention, Treatment and Surveillance of Anthracycline-Induced Cardiovascular Toxicity in Pediatric Cancer Survivors" Hearts 2, no. 1: 45-60. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010005





