Percutaneous Mitral Valve Repair with the MitraClip System in the Current Clinical Practice
Abstract
:1. Mitral Valve Anatomy and Mitral Valve Regurgitation
2. Pathophysiology and Natural History
3. Mitral Regurgitation Assessment and Grading
4. Surgical Treatment of Mitral Regurgitation
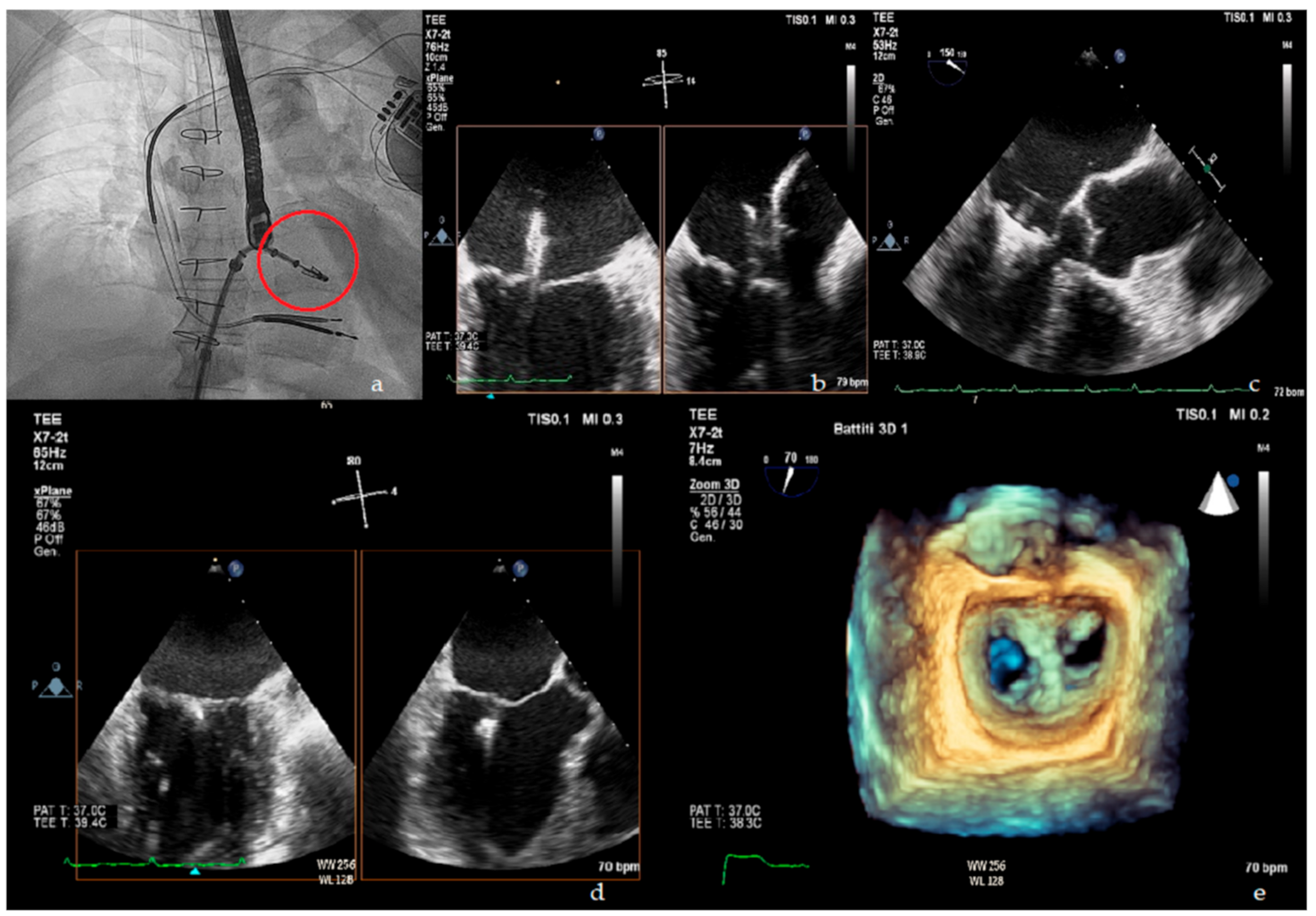
5. Percutaneous Treatment of Mitral Regurgitation
6. MitraClip in the Current Practice for Primary MR
7. MitraClip for Secondary MR Treatment
7.1. COAPT Trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation)
7.2. MITRA–FR
8. Similarities and Differences between COAPT and MITRA–FR
8.1. Medical and Device Therapy at the Baseline
8.2. Echocardiographic Parameters at Baseline
8.3. Procedural Outcomes
9. A New Concept in the Evaluation of MR Severity: Proportionate vs. Disproportionate MR
- Patients whose MR severity is proportionate to the degree of LV dilation and dysfunction (proportionate MR).
- Patients whose MR severity is unexpectedly more compared to their LV dilation and dysfunction (disproportionate MR).
- Patients whose MR, despite an EROA > 20 mm2, is unlikely to be severe given the greater degree of LV dilation (moderate MR).
Controversies of the “Disproportionate MR” Framework
10. Conclusions
11. Open Questions
- Whether proportionate and disproportionate secondary MR represent different stages of the same disease or different clinical entities is unclear.
- A better understanding of pathophysiology of secondary MR could help identify early markers for disproportionate MR and thus prompt treatment.
- Potential diagnostic performance improvement with cardiac MRI needs to be evaluated. Cardiac MRI could overcome the already mentioned limitations and potential underestimation of the PISA method in MR severity assessment, but current guidelines on valvular disease, as well as the trials on MR treatment presented in this review, does not include it in the diagnostic/therapeutic workup; thus it is still not known if a performance improvement in severity assessment could translate into better patients selection and/or better outcomes; moreover, recent studies have shown possible prognostic implications of left ventricular scar extension detected with cardiac magnetic resonance imaging in patients with secondary mitral regurgitation [51].
- Beyond MitraClip: In addition to the MitraClip system, several PMVR systems are currently under investigation. One of these is the Edwards PASCAL system, an edge-to-edge mitral valve repair system that has been shown promising results both in safety and efficacy in the 30 days data of the CLASP study [52], as well as a potential to extend MR treatment to patients who do not fulfill eligibility criteria for MitraClip. Moreover, a further therapeutic approach in the treatment of severe mitral regurgitation is represented by a transcatheter mitral valve replacement (TMVR) treatment; this procedure has emerged as a potential therapy for inoperable or high–surgical risk patients with symptomatic mitral regurgitation. The early feasibility of TMVR has been demonstrated in several prior studies [53], with the Tendyne system (Abbott Structural, Santa Clara, CA, USA) representing the largest experience.
Author Contributions
Funding
Conflicts of Interest
References
- Dal-Bianco, J.P.; Levine, R.A. Anatomy of the mitral valve apparatus: Role of 2D and 3D echocardiography. Cardiol. Clin. 2013, 31, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Sattur, S.; Bates, S.; Movahed, M.R. Prevalence of mitral valve prolapse and associated valvular regurgitations in healthy teenagers undergoing screening echocardiography. Exp. Clin. Cardiol. 2010, 15, e13–e15. [Google Scholar]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, A. Cardiac valve surgery—The “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef]
- Debonnaire, P.; Al Amri, I.; Leong, D.P.; Joyce, E.; Katsanos, S.; Kamperidis, V.; Schalij, M.J.; Bax, J.J.; Marsan, N.A.; Delgado, V. Leaflet remodelling in functional mitral valve regurgitation: Characteristics, determinants, and relation to regurgitation severity. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 290–299. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, K.; Manga, P. Left ventricular remodelling in chronic primary mitral regurgitation: Implications for medical therapy. Cardiovasc. J. Afr. 2018, 29, 51–65. [Google Scholar] [CrossRef]
- Gaasch, W.H.; Meyer, T.E. Left ventricular response to mitral regurgitation: Implications for management. Circulation 2008, 118, 2298–2303. [Google Scholar] [CrossRef] [Green Version]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef]
- Tribouilloy, C.M.; Enriquez-Sarano, M.; Schaff, H.V.; Orszulak, T.A.; Bailey, K.R.; Tajik, A.J.; Frye, R.L. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: Rationale for optimizing surgical indications. Circulation 1999, 99, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Tourneau, T.; Richardson, M.; Juthier, F.; Modine, T.; Fayad, G.; Polge, A.S.; Ennezat, P.V.; Bauters, C.; Vincentelli, A.; Deklunder, G. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010, 96, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Barbieri, A.; Rusinaru, D.; Szymanski, C.; Ferlito, M.; Tafanelli, L.; Bursi, F.; Trojette, F.; et al. MIDA Investigators. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J. Am. Coll. Cardiol. 2009, 54, 1961–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.I.; Igata, S.; Strachan, M.; Nishimura, M.; Wong, D.J.; Raisinghani, A.; DeMaria, A.N. Predictive factors for progression of mitral regurgitation in asymptomatic patients with mitral valve prolapse. Am. J. Cardiol. 2019, 123, 1309–1313. [Google Scholar] [CrossRef]
- Singh, R.G.; Cappucci, R.; Kramer-Fox, R.; Roman, M.J.; Kligfield, P.; Borer, J.S.; Hochreiter, C.; Isom, O.W.; Devereux, R.B. Severe mitral regurgitation due to mitral valve prolapse: Risk factors for development, progression, and need for mitral valve surgery. Am. J. Cardiol. 2000, 85, 193–198. [Google Scholar] [CrossRef]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; Temporelli, P.L. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- Ellis, S.G.; Whitlow, P.L.; Raymond, R.E.; Schneider, J.P. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am. J. Cardiol. 2002, 89, 315–318. [Google Scholar] [CrossRef]
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001, 103, 1759–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Peterson, E.D.; Jacobs, J.P.; He, X.; Brennan, J.M.; O’Brien, S.M.; Dokholyan, R.S.; George, K.M.; Bolling, S.F.; Shahian, D.M.; et al. Longitudinal outcome of isolated mitral repair in older patients: Results from 14,604 procedures performed from 1991 to 2007. Ann. Thorac. Surg. 2012, 94, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Lazam, S.; Vanoverschelde, J.L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.F.; De Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation 2017, 135, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.M.; Vanoverschelde, J.L.; Grigioni, F.; Schaff, H.V.; Tribouilloy, C.; Avierinos, J.F.; Barbieri, A.; Pasquet, A.; Huebner, M.; Rusinaru, D.; et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013, 310, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Rader, F.; Klaar, U.; Gabriel, H.; Krejc, M.; Kalbeck, D.; Schemper, M.; Maurer, G.; Baumgartner, H. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006, 113, 2238–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.V.; Orszulak, T.A.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994, 90, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- van Bommel, R.J.; Marsan, N.A.; Delgado, V.; Borleffs, C.J.W.; van Rijnsoever, E.P.; Schalij, M.J.; Bax, J.J. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation 2011, 124, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Michler, R.E.; Smith, P.K.; Parides, M.K.; Ailawadi, G.; Thourani, V.; Moskowitz, A.J.; Acker, M.A.; Hung, J.W.; Chang, H.L.; Perrault, L.P.; et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N. Engl. J. Med. 2016, 374, 1932–1941. [Google Scholar] [CrossRef]
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.J.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N. Engl. J. Med. 2014, 370, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-year outcomes of surgical treatment of severe ischemic mitralregurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Suradi, H.S.; Kavinsky, C.J.; Hijazi, Z.M. Percutaneous mitral valve repair: The MitraClip device. Glob. Cardiol. Sci. Pract. 2016, 2016, e201617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherif, M.A.; Paranskaya, L.; Yuecel, S.; Kische, S.; Thiele, O.; D’Ancona, G.; Neuhausen-Abramkina, A.; Ortak, J.; Ince, H.; Öner, A. MitraClip step by step; how to simplify the procedure. Neth. Heart J. 2017, 25, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, T.; Kar, S.; Rinaldi, M.; Fail, P.; Hermiller, J.; Smalling, R.; Whitlow, P.L.; Gray, W.; Low, R.; Herrmann, H.C.; et al. Percutaneous mitral repair with the MitraClip system: Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J. Am. Coll. Cardiol. 2009, 54, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attizzani, G.F.; Ohno, Y.; Capodanno, D.; Cannata, S.; Dipasqua, F.; Immé, S.; Mangiafico, S.; Barbanti, M.; Ministeri, M.; Cageggi, A.; et al. Extended use of percutaneous edge-to-edge mitral valve repairbeyond EVEREST (Endovascular Valve Edge-to-Edge Repair) criteria: 30- day and 12-month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc. Interv. 2015, 8 PtA, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Lesevic, H.; Karl, M.; Braun, D.; Barthel, P.; Orban, M.; Pache, J.; Hadamitzky, M.; Mehilli, J.; Stecher, L.; Massberg, S.; et al. Longterm outcomes after MitraClip implantation according to the presence or absence of EVEREST inclusion criteria. Am. J. Cardiol. 2017, 119, 1255–1261. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.; Rahnavardi, M.; Tian, D.H.; Phan, K.; Munkholm-Larsen, S.; Bannon, P.G.; Yan, T.D. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann. Cardiothorac. Surg. 2013, 2, 683–692. [Google Scholar] [CrossRef]
- Pope, N.H.; Lim, S.; Ailawadi, G. Late calcific mitral stenosis after MitraClip procedure in a dialysis-dependent patient. Ann. Thorac. Surg. 2013, 95, e113–e114. [Google Scholar] [CrossRef] [Green Version]
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized comparison of percutaneous repair and surgery for mitralregurgitation: 5-year results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.S.; Reynolds, M.R.; Feldman, T.; Kar, S.; Herrmann, H.C.; Wang, A.; Whitlow, P.L.; Gray, W.A.; Grayburn, P.; Mack, M.J.; et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repair. J. Am. Coll. Cardiol. 2014, 64, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Bonow, R.O. Percutaneous repair of secondary mitral regurgitation—A tale of two trials. N. Engl. J. Med. 2018, 379, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and disproportionate functional mitral regurgitation: A new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Carabello, B.; Hung, J.; Gillam, L.D.; Liang, D.; Mack, M.J.; McCarthy, P.M.; Miller, D.C.; Trento, A.; Siegel, R.J. Defining severe secondary mitral regurgitation:emphasizing an integrated approach. J. Am. Coll. Cardiol. 2014, 64, 2792–2801. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Grayburn, P.A. New Evidence Supporting a Novel Conceptual Framework for Distinguishing Proportionate and Disproportionate Functional Mitral Regurgitation. JAMA Cardiol. 2020, 5, 469–475. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Iung, B.; Armoiry, X.; Trochu, J.N.; Donal, E.; Habib, G.; Brochet, E.; Thibault, H.; Piriou, N.; Cormier, B.; et al. Impact of Mitral Regurgitation Severity and Left Ventricular Remodeling on Outcome After Mitraclip Implantation: Results From the Mitra-FR Trial. JACC Cardiovasc. Imaging 2020. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Sannino, A.; Cohen, D.J.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; Rinaldi, M.J.; Kapadia, S.R.; Rajagopal, V.; et al. Predictors of Clinical Response to Transcatheter Reduction of Secondary Mitral Regurgitation: The COAPT Trial. J. Am. Coll. Cardiol. 2020, 76, 1007–1014. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Packer, M.; Sannino, A.; Stone, G.W. Disproportionate secondary mitral regurgitation: Myths, misconceptions and clinical implications. Heart 2020. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Kusunose, K.; Obuchowski, N.A.; Jellis, C.; Griffin, B.P.; Flamm, S.D.; Kwon, D.H. Prognostic Impact of Ischemic Mitral Regurgitation Severity and Myocardial Infarct Quantification by Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kar, S.; Spargias, K.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.; Fam, N.P.; Walters, D.L.; Webb, J.G.; Smith, R.L.; et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc. Interv. 2019, 12, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
| Leaflet Motion | Lesion | Etiology |
|---|---|---|
| Type I: normal leaflet motion | Annular dilation/distortion Leaflet perforation | Dilated cardiomyopathy, left atrial dilation, Endocarditis |
| Type II: excess leaflet motion (prolapse/flail) | Chordal elongation/rupture Papillary muscle rupture | Degenerative valve disease Ischemic cardiomyopathy, trauma, endocarditis |
| Type IIIA: restricted systo-diastolic leaflet motion | Leaflet and/or chordae thickening/retraction, leaflet calcification/fusion, commissural fusion | Rheumatic heart disease, carcinoid heart disease, dilated cardiomyopathy, radiation |
| Type IIIB: restricted systolic leaflet motion | Papillary muscle displacement or chordal tethering | Ischemic or dilated cardiomyopathy |
| Echocardiographic Parameters | Data/Values Suggestive of Severe MR |
|---|---|
| Qualitative | |
| • Morphologic assessment | Prolapse/flail, chordae or papillary muscle rupture |
| • Color flow MR Jet | Large central jet or eccentric jet reaching the posterior wall of LA |
| • Flow convergence zone | Large flow convergence |
| • CW signal of MR jet | Dense/triangular |
| Semi-quantitative | |
| • Vena contracta width | ≥7mm |
| • Pulmonary vein flow | Systolic flow reversal |
| Quantitative | |
| • EROA | ≥40 mm2 (≥20 mm2 in secondary MR) |
| • Regurgitant volume | ≥60 ml (≥30 ml in secondary MR) |
| • Regurgitant fraction | ≥50% |
| Additional evaluation | |
| • LV and LA size | Chamber dilation (may not be present in acute MR; in secondary MR may be a consequence of underlying LV dysfunction) |
| • Estimated sPAP | >50 mmHg |
| Clinical Setting | Indication for Intervention | Intervention |
|---|---|---|
| Symptomatic chronic primary mitral regurgitation | • LVEF > 30% | Surgery (COR I, LOE B) |
| • LVEF < 30%, LVESD > 55 mmlow surgical risk, no major comorbidities | Repair (COR IIa, LOE C) or Replacement (COR IIb, LOE C) | |
| • LVEF < 30%, LVESD > 55 mmhigh surgical risk and/or major contraindication for surgery | Edge to edge TMVR if feasible (COR IIb, LOE C) | |
| Asymptomatic chronic primary mitral regurgitation | • LVEF < 60% and/or LVESD > 45 mm | Surgery (COR I, LOE B) |
| • LVEF > 60% and new onset AF or sPAP > 50 mmHg | Surgery (COR IIa, LOE B) | |
| • LVEF > 60% + LVESD 40–44 mm and flail leaflet or severe LA dilation; low surgical risk | Repair if high likelihood of durable repair (COR IIa, LOE C) | |
| Symptomatic chronic secondary mitral regurgitation | • LVEF > 30% undergoing CABG | Surgery (COR I, LOE B) |
| • LVEF > 30%, low surgical risk | Surgery (COR IIb, LOE C) | |
| • LVEF < 30% with myocardial viability and option for revascularization | Surgery (COR IIa, LOE C) | |
| • LVEF < 30%, high surgical risk | Edge to edge TMVR if feasible (COR IIb, LOE C); ventricular assist device or transplantation program (COR IIb, LOE C) |
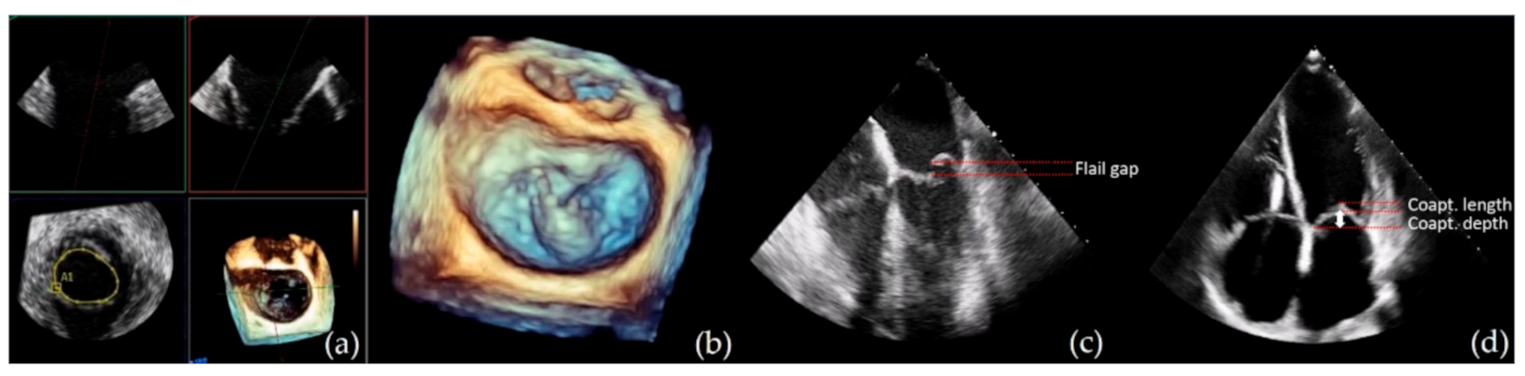
| Parameters | Optimal Suitability | Suboptimal/Conditional Suitability |
|---|---|---|
| Pathology location | A2-P2 | A1-P1 or A3-P3 |
| Calcification | Absent | Mild calcification, not in grasping zone, annular calcification |
| Leaflet mobility | Normal | Systolic restriction |
| Mitral valve area | ≥4 cm2 | ≥3 cm2 |
| Coaptation depth † | <11 mm | ≥11 mm |
| Coaptation length † | ≥2 mm | <2 mm |
| Mobile length of PML | ≥10 mm | 7–10 mm |
| Flail width | ≤15 mm | >15 mm with large annulus size and with the possibility of multiple clip positioning |
| Flail gap | <10 mm |
| Baseline Parameters | COAPT | MITRA–FR |
|---|---|---|
| Etiology of LV dysfunction Ischemic Non-ischemic | 60.7% 39.3% | 59.6% 40.4% |
| LVEDV | 101 ± 34 mL/m2 | 135 ± 35 mL/m2 |
| LVEF inclusion criteria | >20%, <50% | >15%, <40% |
| Mean LVEF | 31% ± 9% | 33 ± 7% |
| EROA cutoff | >30 mm2 | >20 mm2 |
| Mean EROA | 41 ± 15 mm2 | 31 ± 10 mm2 |
| EROA > 30 mm2 | 86% | 48% |
| Additional criteria | LVESD < 70 mm sPAP < 70 mmHg RV dysfunction < moderate |
| Outcome | COAPT | MITRA–FR |
|---|---|---|
| Post-procedural residual MR ≤2 | 95% | 91% |
| 1 year follow up residual ≥3 MR | 5% | 17% |
| % of patients treated with >1 clip | 64% | 54% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, S.; Berardini, A.; Statuto, G.; Angeletti, A.; Massaro, G.; Capobianco, C.; Piemontese, G.P.; Spadotto, A.; Toniolo, S.; Caponetti, A.G.; et al. Percutaneous Mitral Valve Repair with the MitraClip System in the Current Clinical Practice. Hearts 2021, 2, 74-86. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010007
Sorrentino S, Berardini A, Statuto G, Angeletti A, Massaro G, Capobianco C, Piemontese GP, Spadotto A, Toniolo S, Caponetti AG, et al. Percutaneous Mitral Valve Repair with the MitraClip System in the Current Clinical Practice. Hearts. 2021; 2(1):74-86. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010007
Chicago/Turabian StyleSorrentino, Sergio, Alessandra Berardini, Giovanni Statuto, Andrea Angeletti, Giulia Massaro, Claudio Capobianco, Giuseppe Pio Piemontese, Alberto Spadotto, Sebastiano Toniolo, Angelo Giuseppe Caponetti, and et al. 2021. "Percutaneous Mitral Valve Repair with the MitraClip System in the Current Clinical Practice" Hearts 2, no. 1: 74-86. https://0-doi-org.brum.beds.ac.uk/10.3390/hearts2010007






