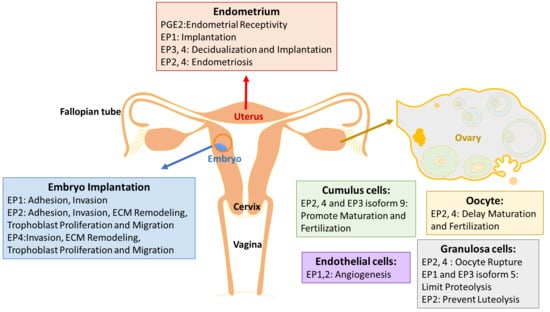
Multiple Roles of Prostaglandin E2 Receptors in Female Reproduction †
Abstract
:1. Introduction
2. EP and PPARγ Receptors Signaling
3. EP Receptors in the Endometrium
Endometriosis
4. EP Receptors in Ovarian Development
5. EP Receptors in the Trophoblast/Embryo
5.1. ECM Remodeling
5.2. Trophoblast Invasion
5.3. Pregnancy Related Diseases
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PG | Prostaglandin |
| AA | arachidonic acid |
| PLA2 | phospholipase A2 |
| PGG2 | prostaglandin G2 |
| PGH2 | prostaglandin H2 |
| COX | cyclooxygenase |
| TXA2 | hromboxane A2 |
| PGES | PGE synthase |
| MRP4 | multidrug resistance protein 4 |
| PGT | prostaglandin transporter |
| GPCRs | G-protein coupled receptors |
| EP | prostaglandin E receptor |
| PPARs | Peroxisome proliferator activated receptors |
| ECL2 | second extracellular loop |
| TMVII | seventh transmembrane domain |
| ICL2 | cond intercellular loop |
| PKC | protein kinase C |
| PLC | phospholipase C |
| FAK | focal adhesion kinase |
| cAMP | cyclic adenosine monophosphate |
| CREB | response element binding protein |
| HIF-1α | hypoxia-inducible factor-1α |
| PI3K | phosphoinositide-3 kinase |
| mTOR | mammalian target of rapamycin |
| ERK | extracellular signal-regulated kinase |
| EGR-1 | early growth response protein 1 |
| AC | adenylyl cyclase |
| RXR | retinoic acid receptor |
| PPPE | peroxisome proliferator response element |
| IL-8 | interleukin-8 |
| RT-PCR | reverse transcription polymerase chain reaction |
| ESCs | endometrial stromal cells |
| Mφ | macrophages |
| NK | natural killer |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| BDNF | brain-derived neurotrophic factor |
| GV | germinal vesicle |
| hOMECs | human ovarian follicular endothelial cells |
| EEC | endometrial epithelial cell |
| TACE | tumor necrosis factor-alpha converting enzyme |
| MT1-MMP | membrane-type 1 matrix metalloproteases |
| HUVECs | Human umbilical vein endothelial cells |
| ECM | extracellular matrix |
References
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers, L.M.; Rees, M.C.; Turnbull, A.C. Arachidonic acid metabolism by the non-pregnant human uterus. Prostaglandins Leukot. Med. 1984, 14, 175–180. [Google Scholar] [CrossRef]
- Kim, S.O.; Duffy, D.M. Mapping PTGERs to the Ovulatory Follicle: Regional Responses to the Ovulatory PGE2 Signal. Biol. Reprod. 2016, 95, 33. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, M.J.; Thaler-Dao, H.; de Paulet, A.C. Prostaglandin synthesis in human placenta and fetal membranes. Prostaglandins 1978, 15, 19–42. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Lala, P.K.; Kennedy, T.G.; Parhar, R.S. Suppression of lymphocyte alloreactivity by early gestational human decidua. II. Characterization of the suppressor mechanisms. Cell. Immunol. 1988, 116, 411–422. [Google Scholar] [CrossRef]
- Clark, J.D.; Lin, L.L.; Kriz, R.W.; Ramesha, C.S.; Sultzman, L.A.; Lin, A.Y.; Milona, N.; Knopf, J.L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 1991, 65, 1043–1051. [Google Scholar] [CrossRef]
- Smith, W.L. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Ann. Rev. Physiol. 1986, 48, 251–262. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef] [Green Version]
- Holla, V.R.; Backlund, M.G.; Yang, P.; Newman, R.A.; DuBois, R.N. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev. Res. 2008, 1, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. The prostaglandin transporter regulates adipogenesis and aromatase transcription. Cancer Prev. Res. 2011, 4, 194–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogacka, I.; Bogacki, M.; Gaglewska, M.; Kurzynska, A.; Wasielak, M. In vitro effect of peroxisome proliferator activated receptor (PPAR) ligands on prostaglandin E2 synthesis and secretion by porcine endometrium during the estrous cycle and early pregnancy. J. Physiol. Pharmacol. 2013, 64, 47–54. [Google Scholar]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Hizaki, H.; Segi, E.; Sugimoto, Y.; Hirose, M.; Saji, T.; Ushikubi, F.; Matsuoka, T.; Noda, Y.; Tanaka, T.; Yoshida, N.; et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc. Natl. Acad. Sci. USA 1999, 96, 10501–10506. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y. Physiological functions of prostanoid receptors and their subtypes. Nihon Yakurigaku Zasshi 2000, 115, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Toh, H.; Ichikawa, A.; Narumiya, S. Molecular evolution of receptors for eicosanoids. FEBS Lett. 1995, 361, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Chen, L.; Chen, X.; He, X.; Yang, J.; Shi, Y.; Zhou, N. The second intracellular loop of the human cannabinoid CB2 receptor governs G protein coupling in coordination with the carboxyl terminal domain. PLoS ONE 2013, 8, e63262. [Google Scholar]
- Negishi, M.; Irie, A.; Sugimoto, Y.; Namba, T.; Ichikawa, A. Selective coupling of prostaglandin E receptor EP3D to Gi and Gs through interaction of alpha-carboxylic acid of agonist and arginine residue of seventh transmembrane domain. J. Biol. Chem. 1995, 270, 16122–16127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillman, B.A.; Audoly, L.; Breyer, R.M. A conserved threonine in the second extracellular loop of the human EP2 and EP4 receptors is required for ligand binding. Eur. J. Pharmacol. 1998, 357, 73–82. [Google Scholar] [CrossRef]
- Ni, W.J.; Tang, L.Q.; Zhou, H.; Ding, H.H.; Qiu, Y.Y. Renoprotective effect of berberine via regulating the PGE2 -EP1-Galphaq-Ca(2+) signalling pathway in glomerular mesangial cells of diabetic rats. J. Cell. Mol. Med. 2016, 20, 1491–1502. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, J.; Guo, Y.; Pan, J.; Yang, Q.; Zhang, M.; Li, H.; Zhang, L.; Ma, J.; Shi, F.; et al. Prostaglandin E2 stimulates beta1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-kappaB pathway. Sci. Rep. 2014, 4, 6538. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.A.; Salinas-Parra, N.; Leach, D.; Navar, L.G.; Prieto, M.C. PGE2 upregulates renin through E-prostanoid receptor 1 via PKC/cAMP/CREB pathway in M-1 cells. Am. J. Physiol. Renal Physiol. 2017, 313, F1038–F1049. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Chou, C.L.; Xu, W.; Chen, X.B.; Woodward, D.F.; Regan, J.W. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of a phosphoinositide-3 kinase signaling pathway. Mol. Pharmacol. 2010, 77, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- Fujino, H.; West, K.A.; Regan, J.W. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 2002, 277, 2614–2619. [Google Scholar] [CrossRef] [Green Version]
- Samuchiwal, S.K.; Balestrieri, B.; Raff, H.; Boyce, J.A. Endogenous prostaglandin E2 amplifies IL-33 production by macrophages through an E prostanoid (EP)2/EP4-cAMP-EPAC-dependent pathway. J. Biol. Chem. 2017, 292, 8195–8206. [Google Scholar] [CrossRef] [Green Version]
- Fujino, H.; Regan, J.W. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol. Pharmacol. 2006, 69, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, H.; Salvi, S.; Regan, J.W. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol. Pharmacol. 2005, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Segi, E.; Sugimoto, Y.; Ichikawa, A.; Negishi, M. Mouse prostaglandin E receptor EP3 subtype mediates calcium signals via Gi in cDNA-transfected Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 1994, 204, 303–309. [Google Scholar] [CrossRef]
- Kotani, M.; Tanaka, I.; Ogawa, Y.; Usui, T.; Tamura, N.; Mori, K.; Narumiya, S.; Yoshimi, T.; Nakao, K. Structural organization of the human prostaglandin EP3 receptor subtype gene (PTGER3). Genomics 1997, 40, 425–434. [Google Scholar] [CrossRef]
- Hatae, N.; Sugimoto, Y.; Ichikawa, A. Prostaglandin receptors: Advances in the study of EP3 receptor signaling. J. Biochem. 2002, 131, 781–784. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Wan, Q.; Zhang, J.; Wang, K.; Shen, Y.; Yu, Y. E-Prostanoid 3 Receptor Mediates Sprouting Angiogenesis Through Suppression of the Protein Kinase A/beta-Catenin/Notch Pathway. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Fujino, H.; Toyomura, K.; Chen, X.B.; Regan, J.W.; Murayama, T. Prostaglandin E(2) regulates cellular migration via induction of vascular endothelial growth factor receptor-1 in HCA-7 human colon cancer cells. Biochem. Pharmacol. 2011, 81, 379–387. [Google Scholar] [CrossRef]
- Namba, T.; Sugimoto, Y.; Negishi, M.; Irie, A.; Ushikubi, F.; Kakizuka, A.; Ito, S.; Ichikawa, A.; Narumiya, S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 1993, 365, 166–170. [Google Scholar] [CrossRef]
- Lalloyer, F.; Staels, B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Salleh, N. Diverse roles of prostaglandins in blastocyst implantation. Sci. World J. 2014, 2014, 968141. [Google Scholar] [CrossRef] [Green Version]
- Logan, P.C.; Ponnampalam, A.P.; Steiner, M.; Mitchell, M.D. Effect of cyclic AMP and estrogen/progesterone on the transcription of DNA methyltransferases during the decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 2013, 19, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Yee, G.M.; Kennedy, T.G. Prostaglandin E2, cAMP and cAMP-dependent protein kinase isozymes during decidualization of rat endometrial stromal cells in vitro. Prostaglandins 1993, 46, 117–138. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Stoikos, C.; Baca, M.; Fairlie, W.D.; McCoubrie, J.E.; Salamonsen, L.A. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J. Clin. Endocrinol. Metab. 2005, 90, 3458–3465. [Google Scholar] [CrossRef] [Green Version]
- Vilella, F.; Ramirez, L.; Berlanga, O.; Martinez, S.; Alama, P.; Meseguer, M.; Pellicer, A.; Simon, C. PGE2 and PGF2alpha concentrations in human endometrial fluid as biomarkers for embryonic implantation. J. Clin. Endocrinol. Metab. 2013, 98, 4123–4132. [Google Scholar] [CrossRef] [Green Version]
- Maybin, J.A.; Hirani, N.; Jabbour, H.N.; Critchley, H.O. Novel roles for hypoxia and prostaglandin E2 in the regulation of IL-8 during endometrial repair. Am. J. Pathol. 2011, 178, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Carson, D.D.; Lagow, E.; Thathiah, A.; Al-Shami, R.; Farach-Carson, M.C.; Vernon, M.; Yuan, L.; Fritz, M.A.; Lessey, B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol. Hum. Reprod. 2002, 8, 871–879. [Google Scholar] [CrossRef]
- Catalano, R.D.; Wilson, M.R.; Boddy, S.C.; Jabbour, H.N. Comprehensive expression analysis of prostanoid enzymes and receptors in the human endometrium across the menstrual cycle. Mol. Hum. Reprod. 2011, 17, 182–192. [Google Scholar] [CrossRef]
- Zhu, J.; Mayr, D.; Kuhn, C.; Mahner, S.; Jeschke, U.; von Schonfeldt, V. Prostaglandin E2 receptor EP1 in healthy and diseased human endometrium. Histochem. Cell Biol. 2018, 149, 153–160. [Google Scholar] [CrossRef]
- Milne, S.A.; Perchick, G.B.; Boddy, S.C.; Jabbour, H.N. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J. Clin. Endocrinol. Metab. 2001, 86, 4453–4459. [Google Scholar] [CrossRef]
- Pakrasi, P.L.; Jain, A.K. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta 2008, 29, 523–530. [Google Scholar] [CrossRef]
- Rahman, M.A.; Li, M.; Li, P.; Wang, H.; Dey, S.K.; Das, S.K. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev. Biol. 2006, 290, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Ma, L.; Ma, W.G.; Maas, R.L.; Dey, S.K. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol. Endocrinol. 1999, 13, 1005–1017. [Google Scholar] [CrossRef]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Yamaguchi, N.; Fujishita, A.; Nakashima, M.; Ishimaru, T.; Masuzaki, H. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Hum. Reprod. 2012, 27, 3417–3424. [Google Scholar] [CrossRef] [Green Version]
- Arosh, J.A.; Lee, J.; Balasubbramanian, D.; Stanley, J.A.; Long, C.R.; Meagher, M.W.; Osteen, K.G.; Bruner-Tran, K.L.; Burghardt, R.C.; Starzinski-Powitz, A.; et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. USA 2015, 112, 9716–9721. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.C.; Ho, H.C. Effects of prostaglandin E2 and vascular endothelial growth factor on sperm might lead to endometriosis-associated infertility. Fertil. Steril. 2011, 95, 360–362. [Google Scholar] [CrossRef]
- Lane, N.E. Pain management in osteoarthritis: The role of COX-2 inhibitors. J. Rheumatol. Suppl. 1997, 49, 20–24. [Google Scholar]
- Gambera, L.; Serafini, F.; Morgante, G.; Focarelli, R.; De Leo, V.; Piomboni, P. Sperm quality and pregnancy rate after COX-2 inhibitor therapy of infertile males with abacterial leukocytospermia. Hum. Reprod. 2007, 22, 1047–1051. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.E.; Berardo, P.T.; Landgraf, R.G.; Fernandes, P.D.; Palmero, C.; Alves, L.M.; Abrao, M.S.; Nasciutti, L.E. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil. Steril. 2010, 93, 2674–2679. [Google Scholar] [CrossRef]
- Chuang, P.C.; Lin, Y.J.; Wu, M.H.; Wing, L.Y.; Shoji, Y.; Tsai, S.J. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am. J. Pathol. 2010, 176, 850–860. [Google Scholar] [CrossRef]
- Takenaka, Y.; Taniguchi, F.; Miyakoda, H.; Takai, E.; Terakawa, N.; Harada, T. Lipopolysaccharide promoted proliferation and invasion of endometriotic stromal cells via induction of cyclooxygenase-2 expression. Fertil. Steril. 2010, 93, 325–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamba, S.; Yodoi, R.; Morimoto, K.; Inazumi, T.; Sukeno, M.; Segi-Nishida, E.; Okuno, Y.; Tsujimoto, G.; Narumiya, S.; Sugimoto, Y. Expression profiling of cumulus cells reveals functional changes during ovulation and central roles of prostaglandin EP2 receptor in cAMP signaling. Biochimie 2010, 92, 665–675. [Google Scholar] [CrossRef]
- Bayne, R.A.; Eddie, S.L.; Collins, C.S.; Childs, A.J.; Jabbour, H.N.; Anderson, R.A. Prostaglandin E2 as a regulator of germ cells during ovarian development. J. Clin. Endocrinol. Metab. 2009, 94, 4053–4060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, D.M.; McGinnis, L.K.; Vandevoort, C.A.; Christenson, L.K. Mammalian oocytes are targets for prostaglandin E2 (PGE2) action. Reprod. Biol. Endocrinol. 2010, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trau, H.A.; Davis, J.S.; Duffy, D.M. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol. Reprod. 2015, 92, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trau, H.A.; Brannstrom, M.; Curry, T.E., Jr.; Duffy, D.M. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum. Reprod. 2016, 31, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Markosyan, N.; Duffy, D.M. Prostaglandin E2 acts via multiple receptors to regulate plasminogen-dependent proteolysis in the primate periovulatory follicle. Endocrinology 2009, 150, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Komar, C.M.; Braissant, O.; Wahli, W.; Curry, T.E., Jr. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 2001, 142, 4831–4838. [Google Scholar] [CrossRef]
- Weems, Y.S.; Nett, T.M.; Rispoli, L.A.; Davis, T.L.; Johnson, D.L.; Uchima, T.; Raney, A.; Lennon, E.; Harbert, T.; Bowers, G.; et al. Effects of prostaglandin E and F receptor agonists in vivo on luteal function in ewes. Prostaglandins Other Lipid Mediat. 2010, 92, 67–72. [Google Scholar] [CrossRef]
- Zhai, J.; Li, S.; Cheng, X.; Chen, Z.J.; Li, W.; Du, Y. A candidate pathogenic gene, zinc finger gene 217 (ZNF217) may contribute to polycystic ovary syndrome through prostaglandin E2. Acta Obstet. Gynecol. Scand. 2020, 99, 119–126. [Google Scholar] [CrossRef]
- Navarra, P.; Andreani, C.L.; Lazzarin, N.; Pierro, E.; Mirtella, A.; Lanzone, A.; Mancuso, S. Increased production and release of prostaglandin-E2 by human granulosa cells from polycystic ovaries. Prostaglandins 1996, 52, 187–197. [Google Scholar] [CrossRef]
- Waclawik, A.; Kaczynski, P.; Jabbour, H.N. Autocrine and paracrine mechanisms of prostaglandin E(2) action on trophoblast/conceptus cells through the prostaglandin E(2) receptor (PTGER2) during implantation. Endocrinology 2013, 154, 3864–3876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, C.; Timoshenko, A.V.; Dixon, S.J.; Lala, P.K.; Chakraborty, C. EP1 receptor-mediated migration of the first trimester human extravillous trophoblast: The role of intracellular calcium and calpain. J. Clin. Endocrinol. Metab. 2005, 90, 4736–4746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horita, H.; Kuroda, E.; Hachisuga, T.; Kashimura, M.; Yamashita, U. Induction of prostaglandin E2 production by leukemia inhibitory factor promotes migration of first trimester extravillous trophoblast cell line, HTR-8/SVneo. Hum. Reprod. 2007, 22, 1801–1809. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Lim, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol. Endocrinol. 2000, 14, 1147–1161. [Google Scholar] [CrossRef]
- Banerjee, P.; Jana, S.K.; Pasricha, P.; Ghosh, S.; Chakravarty, B.; Chaudhury, K. Proinflammatory cytokines induced altered expression of cyclooxygenase-2 gene results in unreceptive endometrium in women with idiopathic recurrent spontaneous miscarriage. Fertil. Steril. 2013, 99, 179–187. [Google Scholar] [CrossRef]
- Woodward, D.F.; Jones, R.L.; Narumiya, S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of prostanoid receptors, updating 15 years of progress. Pharmacol. Rev. 2011, 63, 471–538. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Banu, S.K.; Subbarao, T.; Starzinski-Powitz, A.; Arosh, J.A. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol. Cell. Endocrinol. 2011, 332, 306–313. [Google Scholar] [CrossRef]
- Thathiah, A.; Blobel, C.P.; Carson, D.D. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 2003, 278, 3386–3394. [Google Scholar] [CrossRef] [Green Version]
- Thathiah, A.; Carson, D.D. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem. J. 2004, 382, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef]
- Salgado, R.M.; Covarrubias, A.C.; Favaro, R.R.; Serrano-Nascimento, C.; Nunes, M.T.; Zorn, T.M. Estradiol induces transcriptional and posttranscriptional modifications in versican expression in the mouse uterus. J. Mol. Histol. 2013, 44, 221–229. [Google Scholar] [CrossRef]
- Kershaw-Young, C.M.; Khalid, M.; McGowan, M.R.; Pitsillides, A.A.; Scaramuzzi, R.J. The mRNA expression of prostaglandin E receptors EP2 and EP4 and the changes in glycosaminoglycans in the sheep cervix during the estrous cycle. Theriogenology 2009, 72, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mosher, A.A.; Rainey, K.J.; Giembycz, M.A.; Wood, S.; Slater, D.M. Prostaglandin E2 represses interleukin 1 beta-induced inflammatory mediator output from pregnant human myometrial cells through the EP2 and EP4 receptors. Biol. Reprod. 2012, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biondi, C.; Ferretti, M.E.; Pavan, B.; Lunghi, L.; Gravina, B.; Nicoloso, M.S.; Vesce, F.; Baldassarre, G. Prostaglandin E2 inhibits proliferation and migration of HTR-8/SVneo cells, a human trophoblast-derived cell line. Placenta 2006, 27, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Ryantova, M.; Ulcova-Gallova, Z.; Micanova, Z.; Bibkova, K.; Sediva, B. Levels of prostaglandin E2 (PGE2) in cervical ovulatory mucus in women with spontaneous miscarriages. Ceska Gynekol. 2008, 73, 98–101. [Google Scholar] [PubMed]
- Banerjee, P.; Ghosh, S.; Dutta, M.; Subramani, E.; Khalpada, J.; Roychoudhury, S.; Chakravarty, B.; Chaudhury, K. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS ONE 2013, 8, e80940. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Vattai, A.; Ditsch, N.; Kuhn, C.; Rahmeh, M.; Mahner, S.; Ripphahn, M.; Immler, R.; Sperandio, M.; Jeschke, U.; et al. Prostaglandin E2 receptor 3 signaling is induced in placentas with unexplained recurrent pregnancy losses. Endocr. Connect. 2018, 7, 749–761. [Google Scholar] [CrossRef]
- Kolben, T.M.; Rogatsch, E.; Vattai, A.; Hester, A.; Kuhn, C.; Schmoeckel, E.; Mahner, S.; Jeschke, U.; Kolben, T. PPARgamma Expression Is Diminished in Macrophages of Recurrent Miscarriage Placentas. Int. J. Mol. Sci. 2018, 19, 1872. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, K.; Endo, T.; Kitajima, Y.; Manase, K.; Nagasawa, K.; Honnma, H.; Hayashi, T.; Kudo, R.; Saito, T. Elevation of both cyclooxygenase-2 and prostaglandin E2 receptor EP3 expressions in rat placenta after uterine artery ischemia-reperfusion. Placenta 2006, 27, 395–401. [Google Scholar] [CrossRef]
- El-Bassiouni, E.A.; Helmy, M.H.; Abou Rawash, N.; El-Zoghby, S.M.; Kamel, M.A.; Abou Raya, A.N. Embryopathy in experimental diabetic gestation: Assessment of PGE2 level, gene expression of cyclooxygenases and apoptosis. Br. J. Biomed. Sci. 2005, 62, 161–165. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Lin, P.; Zhu, J.; Jeschke, U.; von Schönfeldt, V. Multiple Roles of Prostaglandin E2 Receptors in Female Reproduction. Endocrines 2020, 1, 22-34. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1010003
Ye Y, Lin P, Zhu J, Jeschke U, von Schönfeldt V. Multiple Roles of Prostaglandin E2 Receptors in Female Reproduction. Endocrines. 2020; 1(1):22-34. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1010003
Chicago/Turabian StyleYe, Yao, Peng Lin, Junyan Zhu, Udo Jeschke, and Viktoria von Schönfeldt. 2020. "Multiple Roles of Prostaglandin E2 Receptors in Female Reproduction" Endocrines 1, no. 1: 22-34. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1010003