Malignancy Analyses of Thyroid Nodules in Patients Subjected to Surgery with Cytological- and Ultrasound-Based Risk Stratification Systems
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. US Assessment
2.3. FNA Cytology Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants and Final Histological Outcomes
3.2. Rates of Malignancy for the ICCRTC Categories
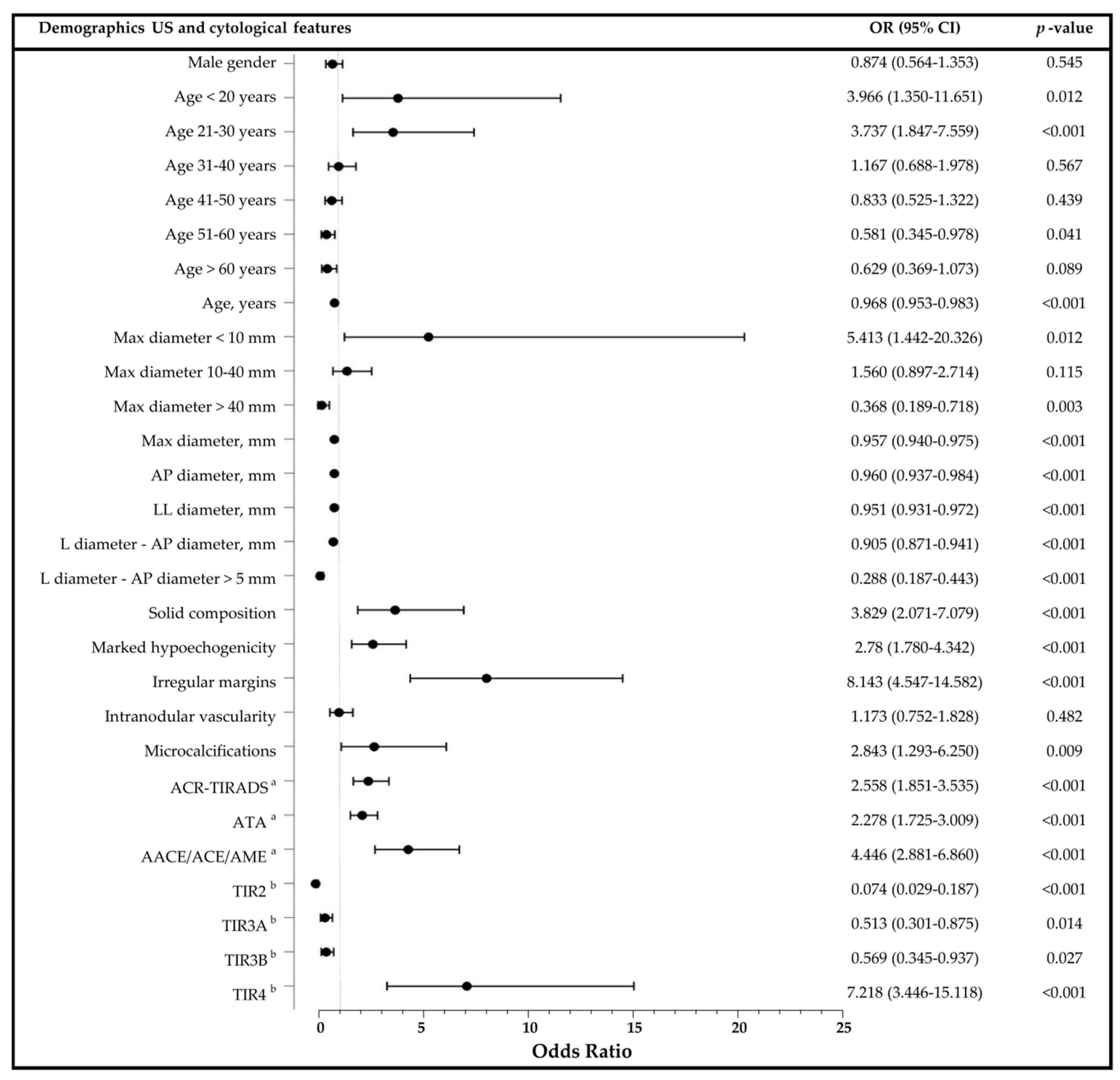
3.3. Predictors of Malignancy in Thyroid Nodules and Diagnostic Performance of US Risk Stratification Systems
3.4. Predictors of Malignancy in Indeterminate Thyroid Nodules
3.5. Predictive Values of a Measured Difference between L and AP Diameter ≤5 mm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P.; AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [Green Version]
- Frates, M.C.; Benson, C.B.; Doubilet, P.M.; Kunreuther, E.; Contreras, M.; Cibas, E.S.; Orcutt, J.; Moore, F.D.; Larsen, P.R.; Marqusee, E.; et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J. Clin. Endocrinol. Metab. 2006, 91, 3411–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegedus, L. Clinical practice. The thyroid nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Medina, J.; Sánchez, J.C.; Viúdez, A.; Grande, E.; Porras, I.; Cajal, T.R.Y.; Trigo, J.; Iglesias, L.; Capdevila, J. SEOM clinical guideline thyroid cancer (2019). Clin. Transl. Oncol. 2020, 22, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.; Newbold, K.; Papotti, M.; Berruti, A. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Moses, W.; Weng, J.; Sansano, I.; Peng, M.; Khanafshar, E.; Ljung, B.-M.; Duh, Q.-Y.; Clark, O.H.; Kebebew, E. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J. Surg. 2010, 34, 2589–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angell, T.E.; Vyas, C.M.; Barletta, J.A.; Cibas, E.S.; Cho, N.L.; Doherty, G.M.; Gawande, A.A.; Howitt, B.E.; Krane, J.F.; Marqusee, E.; et al. Reasons associated with total thyroidectomy as initial surgical management of an indeterminate thyroid nodule. Ann. Surg. Oncol. 2018, 25, 1410–1417. [Google Scholar] [CrossRef]
- Sena, G.; Gallo, G.; Innaro, N.; Laquatra, N.; Tolone, M.; Sacco, R.; Sammarco, G. Total thyroidectomy vs completion thyroidectomy for thyroid nodules with indeterminate cytology/follicular proliferation: A single-centre experience. BMC Surg. 2019, 19, 87. [Google Scholar] [CrossRef]
- Almquist, M.; Muth, A. Surgical management of cytologically indeterminate thyroid nodules. Gland. Surg. 2019, 8 (Suppl. 2), S105–S111. [Google Scholar] [CrossRef]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Crescenzi, A.; Castellana, M.; Giorgino, F.; Giovanella, L.; Bongiovanni, M. Italian consensus for the classification and reporting of thyroid cytology: The risk of malignancy between indeterminate lesions at low or high risk. A systematic review and meta-analysis. Endocrine 2019, 63, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, A.; Pozza, C.; Pofi, R.; Sbardella, E.; Faggiano, A.; Isidori, A.M.; Giannetta, E.; Pernazza, A.; Rullo, E.; Ascoli, V.; et al. Predictors of malignancy in high-risk indeterminate (TIR3B) cytopathology thyroid nodules. J. Endocrinol. Investig. 2020, 43, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Straccia, P.; Santoro, A.; Rossi, E.D.; Brunelli, C.; Mosseri, C.; Musarra, T.; Pontecorvi, A.; Lombardi, C.P.; Fadda, G. Incidence, malignancy rates of diagnoses and cyto-histological correlations in the new Italian Reporting System for Thyroid Cytology: An institutional experience. Cytopathology 2017, 28, 503–508. [Google Scholar] [CrossRef]
- Trimboli, P.; Fulciniti, F.; Merlo, E.; Barizzi, J.; Mazzucchelli, L.; Giovanella, L. Histologic Outcome of Indeterminate Thyroid Nodules Classified at Low or High Risk. Endocr. Pathol. 2018, 29, 75–79. [Google Scholar] [CrossRef]
- Giordano, C.; Barone, I.; Marsico, S.; Bruno, R.; Bonofiglio, D.; Catalano, S.; Andò, S. Endemic Goiter and Iodine Prophylaxis in Calabria, a Region of Southern Italy: Past and Present. Nutrients 2019, 11, 2428. [Google Scholar] [CrossRef] [Green Version]
- Benvenga, S.; Trimarchi, F. Changed presentation of Hashimoto’s thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid 2008, 18, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Minelli, G.; Conti, S.; Manno, V.; Olivieri, A.; Ascoli, V. The geographical pattern of thyroid cancer mortality between 1980 and 2009 in Italy. Thyroid 2013, 23, 1609–1618. [Google Scholar] [CrossRef] [Green Version]
- Witczak, J.; Taylor, P.; Chai, J.; Amphlett, B.; Soukias, J.-M.; Das, G.; Tennant, B.P.; Geen, J.; Okosieme, O.E. Predicting malignancy in thyroid nodules: Feasibility of a predictive model integrating clinical, biochemical, and ultrasound characteristics. Thyroid Res. 2016, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Remonti, L.R.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C.; Gross, J.L. Thyroid ultrasound features and risk of carcinoma: A systematic review and meta-analysis of observational studies. Thyroid 2015, 25, 538–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrabano, P.; McIver, B. Evaluation and Management of Indeterminate Thyroid Nodules: The Revolution of Risk Stratification beyond Cytological Diagnosis. Cancer Control 2017, 24, 1073274817729231. [Google Scholar] [CrossRef]
- Alexander, E.K.; Marqusee, E.; Orcutt, J.; Benson, C.B.; Frates, M.C.; Doubilet, P.M.; Cibas, E.S.; Atri, A. Thyroid nodule shape and prediction of malignancy. Thyroid 2004, 14, 953–958. [Google Scholar] [CrossRef]
- Moon, H.J.; Kwak, J.Y.; Kim, E.K.; Kim, M.J. A taller-than-wide shape in thyroid nodules in transverse and longitudinal ultrasonographic planes and the prediction of malignancy. Thyroid 2011, 21, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Pacini, F.; Basolo, F.; Bellantone, R.; Boni, G.; Cannizzaro, M.A.; De Palma, M.; Durante, C.; Elisei, R.; Fadda, G.; Frasoldati, A.; et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: Joint statements of six Italian societies. J. Endocrinol. Investig. 2018, 41, 849–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grani, G.; Lamartina, L.; Cantisani, V.; Maranghi, M.; Lucia, P.; Durante, C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr. Connect. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: Toward the “right” TIRADS. J. Clin. Endocrinol. Metab. 2019, 104, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Nardi, F.; D’Ambrosio, F.; Rubini, A.; Giacomelli, L.; Biffoni, M.; Filetti, S.; et al. Ultrasonography scoring systems can rule out malignancy in cytologically indeterminate thyroid nodules. Endocrine 2017, 57, 256–261. [Google Scholar] [CrossRef]
- Ciledag, N.; Arda, K.; Aribas, B.K.; Aktas, E.; Köse, S.K. The utility of ultrasound elastography and MicroPure imaging in the differentiation of benign and malignant thyroid nodules. AJR Am. J. Roentgenol. 2012, 198, W244–W249. [Google Scholar] [CrossRef]
- Ying, M.; Bhatia, K.S.; Lee, Y.P.; Yuen, H.Y.; Ahuja, A.T. Review of ultrasonography of malignant neck nodes: Greyscale, doppler, contrast enhancement and elastography. Cancer Imaging 2014, 13, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Lauria Pantano, A.; Maddaloni, E.; Briganti, S.I.; Anguissola, G.B.; Perrella, E.; Taffon, C.; Palermo, A.; Pozzilli, P.; Manfrini, S.; Crescenzi, A. Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur. J. Endocrinol. 2018, 178, 595–603. [Google Scholar] [CrossRef]
- Chiofalo, M.G.; Signoriello, S.; Fulciniti, F.; Avenia, N.; Ristagno, S.; Lombardi, C.P.; Nicolosi, A.; Pelizzo, M.R.; Perigli, G.; Polistena, A.; et al. Predictivity of clinical, laboratory and imaging findings in diagnostic definition of palpable thyroid nodules. A multicenter prospective study. Endocrine 2018, 61, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018, 319, 914–924. [Google Scholar] [CrossRef]
- Girardi, F.M. Thyroid Carcinoma Pattern Presentation According to Age. Int. Arch. Otorhinolaryngol. 2017, 21, 38–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuru, B.; Gulcelik, N.E.; Gulcelik, M.A.; Dincer, H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck 2009, 31, 856–866. [Google Scholar] [CrossRef]
- Vigneri, R.; Malandrino, P.; Vigneri, P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr. Opin. Oncol. 2015, 27, 1–7. [Google Scholar] [CrossRef]
- Moon, H.J.; Kwak, J.Y.; Kim, M.J.; Son, E.J.; Kim, E.K. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology 2010, 255, 260–269. [Google Scholar] [CrossRef]
- Foschini, M.P.; Ragazzi, M.; Parmeggiani, A.L.; Righi, A.; Flamminio, F.; Meringolo, D.; Castaldini, L. Comparison between echo-color Doppler sonography features and angioarchitecture of thyroid nodules. Int. J. Surg. Pathol. 2007, 15, 135–142. [Google Scholar] [CrossRef]
- Kamran, S.C.; Marqusee, E.; Kim, M.I.; Frates, M.C.; Ritner, J.; Peters, H.; Benson, C.B.; Doubilet, P.M.; Cibas, E.S.; Barletta, J.; et al. Thyroid nodule size and prediction of cancer. J. Clin. Endocrinol. Metab. 2013, 98, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, N.A.; White, M.G.; Angelos, P.; Grogan, R.H. Large cytologically benign thyroid nodules do not have high rates of malignancy or false-negative rates and clinical observation should be considered: A meta-analysis. Thyroid 2018, 28, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Bestepe, N.; Ozdemir, D.; Baser, H.; Ogmen, B.; Sungu, N.; Kilic, M.; Ersoy, R.; Cakir, B. Is thyroid nodule volume predictive for malignancy? Arch. Endocrinol. Metab. 2019, 63, 337–344. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, W.; Yang, J.; Yang, J.; Shao, K.; Yuan, L.; Chen, H.; Lu, W.; Zhu, Y. Multidetector computed tomography analysis of benign and malignant nodules in patients with chronic lymphocytic thyroiditis. Oncol. Lett. 2016, 12, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Liu, M.; He, J.; Wu, S.; Chen, M.; Wan, Y.; Gao, L.; Cai, X.; Ding, J.; Fu, X. Comparison of Different Risk-Stratification Systems for the Diagnosis of Benign and Malignant Thyroid Nodules. Front. Oncol. 2019, 9, 378. [Google Scholar] [CrossRef]
- Kalovidouris, A.A. US Findings in Head and Neck Cancer. In Imaging in Clinical Oncology; Gouliamos, A., Andreou, J., Kosmidis, P., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Trimboli, P.; Fulciniti, F.; Zilioli, V.; Ceriani, L.; Giovanella, L. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn. Cytopathol. 2017, 45, 113–117. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, E.K.; Kim, M.J.; Kim, B.M.; Oh, K.K.; Hong, S.W.; Park, C.S. Ultrasonographic characteristics of subacute granulomatous thyroiditis. Korean J. Radiol. 2006, 7, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Jung, C.K.; Lee, J.H. Degenerating thyroid nodules: Ultrasound diagnosis, clinical significance, and management. Korean J. Radiol. 2019, 20, 947–955. [Google Scholar] [CrossRef]
- Lee, H.Y.; Baek, J.H.; Ha, E.J.; Park, J.W.; Lee, J.; Song, D.E.; Shong, Y.K. Malignant-looking thyroid nodules with size reduction: Core needle biopsy results. Ultrasonography 2016, 35, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; McManus, C.; Chen, H.; Sippel, R.S. Are there predictors of malignancy in patients with multinodular goiter? J. Surg. Res. 2012, 174, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Baek, J.H.; Hong, M.J.; Lee, J.H. Inter-observer variation in ultrasound measurement of the volume and diameter of thyroid nodules. Korean J. Radiol. 2015, 16, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boi, F.; Minerba, L.; Lai, M.L.; Marziani, B.; Figus, B.; Spanu, F.; Borghero, A.; Mariotti, S. Both thyroid autoimmunity and increased serum TSH are independent risk factors for malignancy in patients with thyroid nodules. J. Endocrinol. Investig. 2013, 36, 313–320. [Google Scholar] [CrossRef]

| Demographic Characteristics | Benign Nodules (N = 247) | Malignant Nodules (N = 153) | p-Value |
|---|---|---|---|
| Female gender, N | 166 (67.2%) | 105 (68.6) | 0.826 |
| Age, years | 49 ± 13 | 43 ± 15 | <0.001 |
| Age < 20 years, N | 5 (2%) | 11 (7.2%) | 0.016 |
| Age 21–30 years, N | 12 (4.9%) | 26 (17%) | <0.001 |
| Age 31–40 years, N | 41 (16.6%) | 26 (17%) | 1.0 |
| Age 41–50 years, N | 72 (29.1%) | 40 (26.1%) | 0.567 |
| Age 51–60 years, N | 62 (25.1%) | 25 (16.3%) | 0.046 |
| Age > 60 years, N | 55 (22.3%) | 24 (15.7%) | 0.122 |
| US Characteristics | Benign Nodules (N = 247) | Malignant Nodules (N = 153) | p-Value |
|---|---|---|---|
| Position, N | |||
| Right Lobe | 112 (45.3%) | 68 (44.4%) | 0.918 |
| Left Lobe | 120 (48.6%) | 70 (45.8%) | 0.607 |
| Isthmus | 15 (6.1%) | 9 (5.9%) | 1.0 |
| AP diameter, mm | 17 (12.5–24.7) | 13.3 (10.0–20.9) | <0.001 |
| LL diameter, mm | 22.5 (16.2–32.0) | 15.6 (11.6–24.3) | <0.001 |
| L diameter, mm | 26.3 (19.0–35.8) | 18.4 (12.7–28.1) | <0.001 |
| Max diameter, mm | 26.3 (19.3–36.8) | 18.5 (12.6–28.2) | <0.001 |
| Max diameter, N | |||
| <10 mm | 3 (1.2%) | 9 (5.9%) | 0.013 |
| 10–40 mm | 197 (79.8%) | 129 (84.3%) | 0.290 |
| >40 mm | 47 (19%) | 14 (9.2%) | 0.010 |
| Volume, mm3 | 5956 (2262–14,168) | 1670 (764–7432) | <0.001 |
| Surface area, mm2 | 1611 (863–3062) | 689 (419–1873) | <0.001 |
| Shape, N | |||
| Round | 3 (1.2%) | 2 (1.4%) | 1.0 |
| Oval | 208 (84.2%) | 99 (68.3%) | <0.001 |
| Taller-than-wide | 5 (2%) | 8 (5.5%) | 0.163 |
| Composition, N | |||
| Solid | 176 (71.3%) | 138 (90.2%) | <0.001 |
| Mixed | 66 (26.7%) | 15 (9.8%) | <0.001 |
| Cystic | 3 (1.2%) | 0 (0%) | 0.290 |
| Spongiform | 2 (0.8%) | 0 (0%) | 0.526 |
| Echogenicity, N | |||
| Isoechoic | 58 (24.3%) | 26 (17.3%) | 0.131 |
| Hypoechoic | 127 (53.1%) | 57 (38%) | 0.007 |
| Marked Hypoechoic | 52 (21.8%) | 66 (44%) | <0.001 |
| Hyperechoic | 0 (0%) | 1 (0.7%) | 0.383 |
| Anechoic | 2 (0.8%) | 0 (0%) | 0.527 |
| Margins, N | |||
| Well-defined | 231 (93.5%) | 125 (81.7%) | <0.001 |
| Ill-defined | 16 (6.5%) | 28 (18.3%) | <0.001 |
| Regular | 229 (92.7%) | 96 (62.7%) | <0.001 |
| Irregular | 18 (7.3%) | 57 (37.3%) | <0.001 |
| Vascularity, N | |||
| Absent | 63 (25.5%) | 29 (19%) | 0.143 |
| Perinodular | 38 (15.4%) | 20 (13.1%) | 0.562 |
| Intranodular | 2 (0.8%) | 3 (2%) | 0.376 |
| Peri-intranodular | 66 (26.7%) | 46 (30.1%) | 0.492 |
| Calcifications, N | |||
| Microcalcifications | 11 (4.5%) | 17 (11.1%) | 0.015 |
| Macrocalcifications | 16 (6.5%) | 14 (9.1%) | 0.335 |
| Peripheral-rim | 0 (0%) | 2 (1.3%) | 146 |
| Suspicious lymph nodes, N | 2 (0.8%) | 14 (9.2%) | <0.001 |
| US risk category, N | |||
| AACE/ACE/AME | |||
| Low | 4 (1.6%) | 0 (0%) | 0.303 |
| Intermediate | 159 (64.4%) | 50 (32.7%) | <0.001 |
| High | 77 (31.2%) | 102 (66.7%) | <0.001 |
| ATA | |||
| Benign | 3 (1.2%) | 0 (0%) | 0.290 |
| Very-low suspicion | 13 (5.3%) | 4 (2.6%) | 0.308 |
| Low suspicion | 83 (33.6%) | 29 (19%) | 0.002 |
| Intermediate suspicion | 108 (43.7%) | 56 (36.6%) | 0.175 |
| High suspicion | 33 (13.4%) | 61 (39.9%) | <0.001 |
| ACR-TIRADS | |||
| TR1: benign | 3 (1.2%) | 0 (0%) | 0.290 |
| TR2: not-suspicious | 11 (4.5%) | 4 (2.6%) | 0.425 |
| TR3: mildly suspicious | 72 (29.1%) | 24 (15.7%) | 0.003 |
| TR4: moderately suspicious | 136 (55.1%) | 75 (49.0%) | 0.258 |
| TR5: highly suspicious | 17 (6.9%) | 47 (30.7%) | <0.001 |
| ICCRTC Categories | Benign Nodules (N = 247) | Malignant Nodules (N = 153) | Rates of Malignancy | p-Value |
|---|---|---|---|---|
| Non-diagnostic—TIR1 | 20 (7.3%) | 5 (3.2%) | 17.9% | 0.057 |
| Non diagnostic (cystic)—TIR1C | 3 (2.0%) | 0 (0%) | 0.289 | |
| Benign—TIR2 | 80 (32.4%) | 6 (3.9%) | 7.0% | <0.001 |
| Low-risk indeterminate—TIR3A | 64 (25.9%) | 22 (14.3%) | 25.6% | 0.008 |
| High-risk indeterminate—TIR3B | 69 (27.9%) | 29 (19%) | 29.6% | 0.043 |
| Suspicious of malignancy—TIR4 | 10 (4.0%) | 34 (22.2%) | 77.3% | <0.001 |
| Malignant—TIR5 | 1 (0.4%) | 57 (37.2%) | 98.3% | <0.001 |
| Gender | US Risk-Stratification Systems | OR (95%CI) | p-Value |
|---|---|---|---|
| Female | ACR-TIRADS | 3.053 (2.041–4.565) | <0.001 |
| ATA | 2.334 (1.674–3.255) | <0.001 | |
| AACE/ACE/AME | 4.408 (2.615–7.430) | <0.001 | |
| Male | ACR-TIRADS | 1.954 (1.086–3.514) | 0.025 |
| ATA | 2.418 (1.406–4.158) | 0.001 | |
| AACE/ACE/AME | 4.632 (2.112–10.158) | <0.001 |
| ICCRCT Categories | Demographic and US Features | OR (95%CI) | p-Value |
|---|---|---|---|
| All indeterminate nodules | Age | 0.986 (0.963–1.008) | 0.214 |
| L diameter-AP diameter > 5 mm | 0.386 (0.197–0.754) | 0.005 | |
| Solid composition | 4.091 (1.373–12.185) | 0.011 | |
| Marked hypoechogenicity | 1.550 (0.779–3.082) | 0.212 | |
| Irregular margins | 3.590 (1.361–9.465) | 0.010 | |
| Low-risk indeterminate TIR3A | Age | 1.021 (0.986–1.057) | 0.237 |
| L diameter-AP diameter > 5 mm | 0.467 (0.175–1.248) | 0.129 | |
| Solid composition | 2.800 (0.583–13.455) | 0.199 | |
| Marked hypoechogenicity | 0.917 (0.309–2.718) | 0.875 | |
| Irregular margins | 1.863 (0.407–8.535) | 0.423 | |
| High-risk indeterminate TIR3B | Age | 0.957 (0.927–0.989) | 0.009 |
| L diameter-AP diameter > 5 mm | 0.315 (0.125–0.795) | 0.014 | |
| Solid composition | 5.612 (1.221–25.804) | 0.027 | |
| Marked hypoechogenicity | 2.275 (0.910–5.690) | 0.079 | |
| Irregular margins | 5.862 (1.557–22.080) | 0.009 |
| Cut-off | SEN | SPE | PPV | NPV | Accuracy (95%CI) |
|---|---|---|---|---|---|
| ICCRCT risk category ≥ TIR3B a | 79.1 | 67.1 | 58.5 | 84.5 | 71.5 (66.8–75.9) |
| ICCRCT risk category ≥ TIR3B b | 56.9 | 48.2 | 27.7 | 74.4 | 50.5 (43.1–58.0) |
| L diameter-AP diameter ≤ 5 mm a | 59.6 | 70.2 | 52.8 | 75.6 | 66.4 (61.5–71.0) |
| L diameter-AP diameter ≤ 5 mm b | 53.1 | 69.7 | 38.8 | 80.3 | 65.2 (57.9–72.1) |
| L diameter-AP diameter ≤ 5 mm c | 54.6 | 64.1 | 34.3 | 80.4 | 61.6 (50.5–71.9) |
| L diameter-AP diameter ≤ 5 mm d | 51.9 | 74.7 | 43.8 | 80.3 | 68.4 (58.2–77.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, S.; Mirabelli, M.; Chiefari, E.; Vergine, M.; Gervasi, R.; Brunetti, F.S.; Innaro, N.; Donato, G.; Aversa, A.; Brunetti, A. Malignancy Analyses of Thyroid Nodules in Patients Subjected to Surgery with Cytological- and Ultrasound-Based Risk Stratification Systems. Endocrines 2020, 1, 102-118. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1020010
Giuliano S, Mirabelli M, Chiefari E, Vergine M, Gervasi R, Brunetti FS, Innaro N, Donato G, Aversa A, Brunetti A. Malignancy Analyses of Thyroid Nodules in Patients Subjected to Surgery with Cytological- and Ultrasound-Based Risk Stratification Systems. Endocrines. 2020; 1(2):102-118. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1020010
Chicago/Turabian StyleGiuliano, Stefania, Maria Mirabelli, Eusebio Chiefari, Margherita Vergine, Rita Gervasi, Francesco S. Brunetti, Nadia Innaro, Giuseppe Donato, Antonio Aversa, and Antonio Brunetti. 2020. "Malignancy Analyses of Thyroid Nodules in Patients Subjected to Surgery with Cytological- and Ultrasound-Based Risk Stratification Systems" Endocrines 1, no. 2: 102-118. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines1020010








