1. Introduction
Nowadays, more and more physicians, including gynecologists, are becoming aware of the tremendous impact of various types of stress has on women’s health, particularly on the reproductive system. Stress related to insufficient nutrition, excessive physical effort and psychological experiences have a negative impact not only on reproduction, but on the entire human body. Functional hypothalamic amenorrhea (FHA) is the term used to describe lack of menstruation resulting from various kinds of stress, diagnosed after excluding other etiologies of amenorrhea. It is a common cause of hypogonadotropic hypogonadism in women, occurring in the absence of structural lesion. FHA is responsible for approximately 30% of secondary amenorrhea in women of reproductive age and approximately 3% of primary amenorrhea [
1,
2].
The origin of FHA is believed to rely on impaired pulsatile gonadotropin releasing hormone (GnRH) secretion. Abnormal GnRH secretion causes a reduction in the pulses of both the luteinizing hormone (LH) and follicle stimulating hormone (FSH). As a consequence, there is no increase in LH secretion in the middle of the menstrual cycle, a lack of proper follicular development, successive anovulation and a low serum estradiol (E2) concentration [
3]. It is said that FHA may have a genetic predisposition, such as heterozygosity for congenital hypogonadotropic hypogonadism [
4]. Hypoestrogenism that results from the inhibition of the hypothalamus-pituitary- ovarian (HPO) axis has profound effects on many systems throughout the body, including reproductive, cardiac, skeletal and psychological. That is why it is so important to apply appropriate treatment as soon as possible to prevent numerous short- and long-term health consequences [
5].
Initial management should focus on treating the underlying cause, that is, trying to gain weight, limiting exercise, or avoiding emotional stress. In situations where such treatment is not effective, hormone replacement therapy should be used to maintain normal estrogen levels, or if the patient wishes to become pregnant, treatment focused on ovulation induction or in vitro fertilization (IVF) [
6].
2. Patophysiology
The mechanisms responsible for the development of FHA are not fully understood and still seem unclear. Certainly, numerous neurotransmitters and neurosteroids play an important role in the regulation of the hypothalamic-pituitary-ovarian axis, therefore they most likely also play a role in the pathophysiology of FHA. The most important seem to be kisspeptin, leptin, beta-endorphin, neuropeptide Y (NPY), ghrelin, and corticotropin releasing hormone (CRH). Kisspeptin, which is a product of the KiSS-1 gene and its G. The receptor coupled to the GPR54 protein plays a key role in regulation of reproductive functions. Kisspeptin/GPR54 the system stimulates the hypothalamic-pituitary-ovary axis, but can also directly stimulate the secretion of GnRH from the hypothalamus [
7].
The 2017 Endocrine Society Clinical Practice Guidelines on FHA stress that FHA is a form of chronic ovulation caused not by an organic cause, but by various types of stress, resulting from weight loss, excessive physical exertion or traumatic psychological experiences [
2].
First of all, the influence of different kinds of stress may negatively affect reproduction along the entire hypothalamic-pituitary-ovarian axis. FHA is characterized by a disturbance in pulsatile GnRH secretion, leading to a reduction in the amplitude and/or frequency of gonadotropin pulses and anovulation. Varying neuroendocrine patterns of LH secretion can be observed in women with FHA. Regarding serum FSH levels, low or normal levels are reported, but often above LH levels [
8]. Low energy availability due to increased caloric expenditure and/or insufficient caloric intake can have a negative inhibitory effect on the HPO axis, redirecting energy from reproductive processes to more vital systems for survival [
9].
Additionally, the effect on the axis of growth hormone and insulin-like growth factor 1 (GH- IGF-1) in a situation of energy deficiency, either due to reduced caloric consumption, excessive energy expenditure or both, is a reduced IGF-1 level, despite an increase in GH levels, as a result of a nutritionally acquired resistance to GH [
10].
Another hormone axis that suffers from FHA is the adrenal axis. Strong stressful situations can interact by activating the hypothalamus-pituitary-adrenal (HPA) axis [
11]. This activation is associated with increased secretion of the hypothalamus of the corticotropin releasing hormone (CRH), corticotropin (ACTH) and adrenal cortisol. CRH works by inhibiting the frequency of GnRH pulses, while cortisol inhibits reproductive function at the hypothalamus, pituitary and uterine levels. Therefore, in women diagnosed with FHA, we most often observe higher basal cortisol levels and diminished ACTH and cortisol responses to CRH stimulation. This is most likely secondary to the negative feedback associated with hypercortisolemia and elevated ACTH levels [
12].
Kondoh Y. et al. in a study published in 2001, assessed disorders of the HPA axis in women diagnosed with FHA. Researchers studied 24 women diagnosed with progestin-negative hypothalamic amenorrhea, administered with human corticotropin-releasing hormone (hCRH) and treated with estrogen-progestogen therapy. Researchers focused their attention on both cortisol and ACTH assays and the time needed to recover. Basal cortisol levels were statistically significantly higher in patients with FHA. On the other hand, the concentrations of both cortisol and ACTH observed 30 and 60 min after hCRH administration were statistically significantly lower in patients with FHA compared to healthy women. Additionally, in this study a positive correlation was found between basal cortisol levels in patients with FHA and the time needed for recovery [
13].
The numerous hormones that regulate energy intake or expenditure are also affected by FHA. Low energy availability is associated with changes in the concentrations of many hormones that serve as peripheral metabolic signals to regulate reproductive function, as well as appetite and satiety, and thus energy consumption, or reduce energy expenditure. In patients with FHA, increased levels of the appetite-stimulating hormone ghrelin were observed, secreted by the oxyntic cells of the gastric fundus. This could most likely be an adaptive action to stimulate caloric intake in states of malnutrition. Elevated ghrelin concentrations may impair GnRH secretion and lead to hypogonadism [
14]. Patients with FHA are also characterized by lower levels of leptin, adipokine, which is secreted by fat cells. It is an appropriate adaptive response to encourage food intake in malnutrition conditions. Under physiological conditions, leptin has a positive effect on the secretion of GnRH, thus it affects the entire HPO axis [
15]. Additionally, energy deficit states during FHA, leads to disorders of the hypothalamic-pituitary-thyroid axis, causing hypothyroidism by suppressing the thyroid axis. As a consequence, it has an impact on lowering the basic metabolism. In patients with FHA, we observe thyroid-stimulating hormone (TSH) at an unchanged level, while particularly triiodothyronine (T3) is decreed and free thyroxine (T4) present normal to low levels. Therefore, resting energy expenditure is low in states of low energy availability. Hypothyroidism can lead to impaired bone formation and growth retardation due to the negative effects of the thyroid gland bone metabolism deficiency. The rate of bone formation is reduced by 50%, while the rate of bone resorption is reduced by 40%, leading to greater bone loss. [
16].
Patients who practice sports intensively for long periods are at an increased risk of developing FHA. The “female athlete triad” is the coexistence of lower energy availability, disorders of the menstrual cycle and reduced bone mineral density. All three of these “female athlete triad” factors are becoming more and more common among competitive athletes, especially among those who practice strenuous sports such as competitive running, swimming or ballet, in which there is a particular emphasis on thinness. Athletes have more stress-related fractures and menstrual cycle disorders compared to what is seen in non-athletes. Women suffering from the “female athlete triad” are more likely to experience the long-term consequences of FHA, such as decreased bone mineral density. Therefore, it is so important to develop awareness among this group about the negative effects of excessive exercise, stress and disturbed diet [
17].
An interesting point is that there is considerable variability in the degree of weight loss or physical exertion necessary to induce amenorrhea. This provides grounds for considering the genetic predisposition of individual women to develop FHA. Many gene mutations such as (KAL1, FGFR1, PROKR2, GNRHR) have been identified in patients with congenital GnRH deficiency. Rare variants exist in the genes associated with idiopathic hypogonadotrophic hypogonadism in FHA patients. This suggests that individual mutations may contribute to a greater or lesser susceptibility of women to functional changes, such as exercise, stress or weight loss. It follows from this that depending on individual mutations and genetic conditions, sensitivity to the inhibition of the hypothalamic—pituitary—gonadal axis by such stressors varies substantially. However, it is not known exactly whether this susceptibility reflects a genetic predisposition to hypothalamic amenorrhea [
4].
3. Diagnosis of FHA
The diagnosis of FHA is associated with amenorrhea associated with low serum levels of gonadotropins and hypoestrogenism. Additionally, it is essential to find the cause of these symptoms, i.e., a stress factor such as low body weight, exercise or emotional stress. FHA diagnosis is made by exclusion, and it is very important to rule out other causes of amenorrhea.
The assessment of a patient with FHA should focus on a thorough medical history and gynecological examination. In particular, the interview must include gathering information on the relationship between disturbances in the menstrual cycle and weight loss, overexertion or experienced work- or study-related stress and personal stress, in addition to psychiatric disorders such as anxiety and depression.
The next step in the appropriate evaluation of patients with FHA is biochemical testing. In addition to excluding pregnancy and testing for hCG levels, thyroid-stimulating hormone (TSH), free thyroxine (T4), prolactin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and antiMullerian hormone (AMH) should be measured. Additionally, complete blood count, electrolytes, glucose, bicarbonate, blood urea nitrogen, creatinine, liver panel, and (when appropriate) sedimentation rate and/or C-reactive protein levels should be performed. If symptoms of hyperandorgenism are present, androgen testing should be performed. After excluding pregnancy, administering a progestin challenge in patients with FHA is recommended to induce withdrawal bleeding (as an indication of chronic estrogen exposure) and ensure the integrity of the outflow tract.
Imaging studies in patients with FHA include transabdominal or transvaginal ultrasound and magnetic resonance imaging (MRI) of the sella region in cases of unexplained hypogonadotropic hypogonadism.
Another essential test that should not be forgotten is bone densitometry. If the amenorrhea lasts more than six months or in patients with an additional risk factor of low bone mineral density (BMD) (significant nutritional deficiencies or eating disorders), dual-energy x-ray absorptiometry (DXA) should be performed to indicate baseline BMD and to help guide and monitor treatment [
18].
Due to the possibility of mood disorders in patients with FHA, tests to assess anxiety and mood disorders should be performed.
Standard fertility assessments should be performed by all patients with FHA.
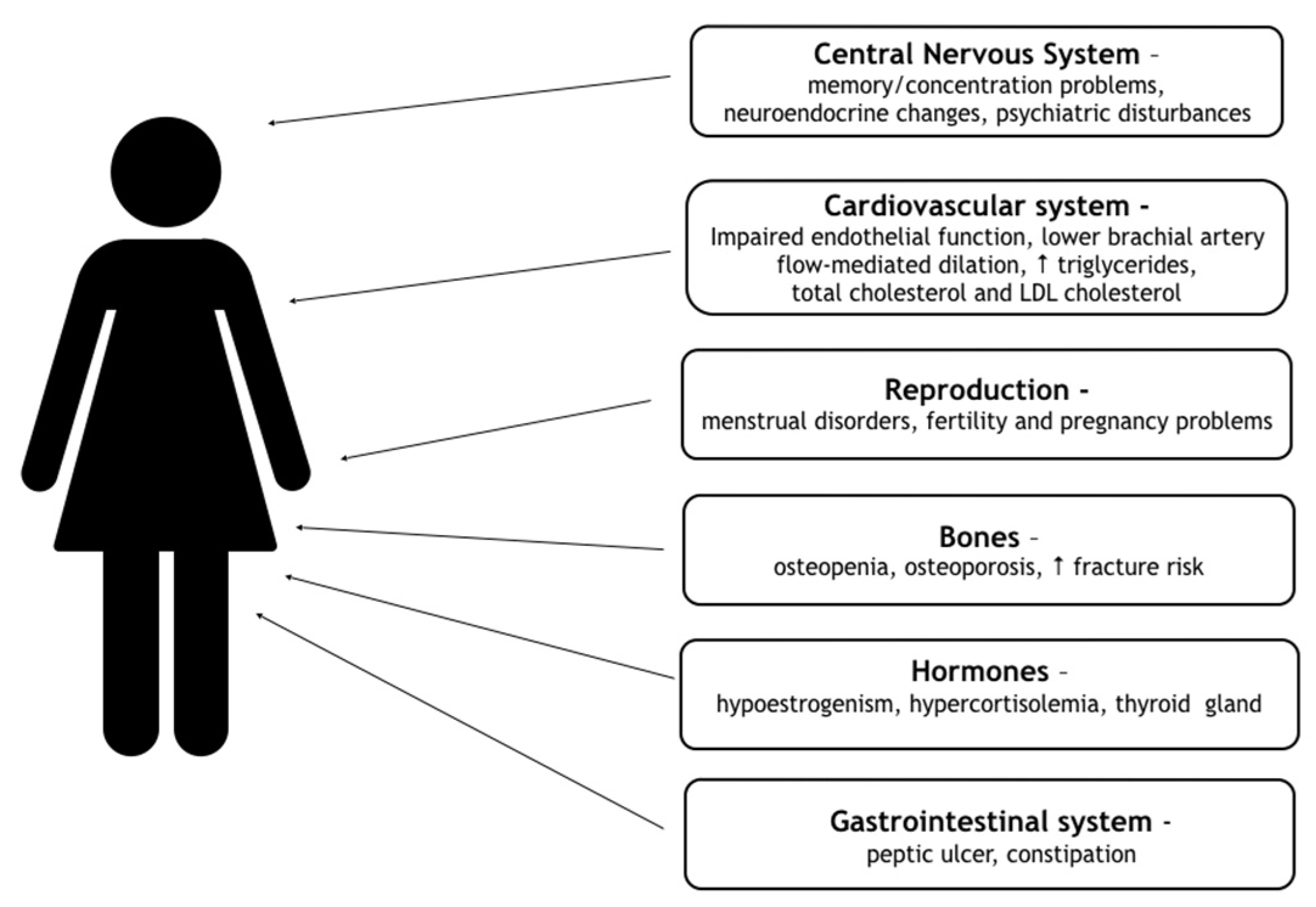
4. Consequences of FHA
Women with FHA are exposed to numerous health consequences of hypoestrogenism. They have a greater risk of developing complications in the reproductive, cardiovascular, skeletal system and psychiatric comorbidities, including anxiety and mood disorders. All consequences are contained in
Figure 1 [
19].
4.1. Reproduction and Pregnancy
FHA-related hypothalamic dysfunction causes GnRH reduction or inhibition secretion, which affects the pulsatile release of pituitary gonadotrophins, causing estrogen deficiency and anovulation. Anovulation and the resulting infertility render patients with FHA unable to become pregnant spontaneously. Reproductive disturbances due to FHA are reversible, and resolve over time after normalization of the patient’s energy availability or resolution of the emotional stress causing FHA. Excessive exercise in women can lead to disorders in the luteal phase, disrupting the implantation and proper development of the developing embryo [
19].
Low estrogen concentration can lead to atrophic changes in the urogenital mucosa and in the uterus causing vaginal dryness and dyspareunia. An increased vaginal pH level predisposes the genitourinary tract to infection and mechanical weakness which usually disappear as estrogen levels normalize and energy availability improves.
In women with FHA, despite the fact that anovulation is the most common, if fertilization has already occurred, numerous complications are very common. FHA patients who have emotional stress and low body mass index (BMI) have an increased risk of miscarriage and preterm labor, in women who become pregnant either spontaneously or after fertility treatment. The mental health of the mother is of great importance for the proper development of the fetus. Inadequate nutrition, strong stressful experiences and the mother’s emotional poor health directly translate into low birth weight [
20,
21].
4.2. Cardiovascular System
The most common cause of death for women in developed countries is cardiovascular disease (CVD). Undoubtedly, inadequate estrogen levels disturb the functioning of the cardiovascular system. Estrogen receptors are found in both coronary and peripheral vessels, which means that estrogen plays a huge role in the regulation of blood vessel function. The stimulating effect of estrogens on nitric oxide, which causes vasodilation, is known. Estrogens exert a cardioprotective effect by affecting the endothelium, blood vessels, directly on the heart muscle and additionally on metabolic parameters. Conditions accompanied by hypoestrogenism can lead to endothelial dysfunction, disturbed bioactivity of nitric oxide and disturbed autonomic function. Additionally, we observe the influence of decreased estrogen concentration on the excessive activation of the renin-angiotensin system and negative changes in the lipid profile [
22].
In terms of the cardiovascular system in patients with FHA with prolonged exercise-induced amenorrhea, negative consequences are observed, including effects on lipid levels, endothelial function and on angiographic evidence of coronary artery disease. Pre-menopausal women with a history of FHA have increased risk of developing cardiovascular disease exhibit impaired endothelial function, which may contribute to impaired vascular function. The effect on lipids is characterized by the observed increased concentration of total cholesterol, triglycerides (TAG), and LDL cholesterol [
23]. In the study of Nicholas Smith et al., a comparison of cardiovascular safety of oral hormone therapy products in postmenopausal women was performed. The scientists proved that oral conjugated equine estrogens (CEEs) use was associated with a higher risk of incident venous thrombosis and possibly myocardial infarction than estradiol use [
24].
An early clinical sign of hypoestrogenemia is menstrual cycle irregularities. Prior research from the Nurses’ Health Study of over 82,000 women that self-reported menstrual cycle history demonstrated that the more irregular the menstrual cycle in young women, the greater the risk for future CVD events; up to a 50% increase [
25].
4.3. Bones
In patients with FHA, the major skeletal problem is impaired bone growth during adolescence, and low bone density in adulthood [
26]. Despite the positive effects of exercise on bone, women with exercise-induced FHA, and particularly those with low body weight or restrictive eating habits, may have decreased bone mineral density. Women with FHA who exercise excessively in adolescence and young adulthood have lower BMD
Z-scores in the lumbar spine compared to normal menstruating women. In these women, bone microarchitecture is impaired and therefore estimated bone strength is reduced [
26]. Fractures, especially overload fractures, are much more common in patients with exercise-induced amenorrhea, confirming the reduced bone strength in this group of women. Overload fractures are much more common in athletes with disturbed eating patterns compared to those who eat properly. This may be due in part to low bone mass, but also a low energy state leading to bone resorption and low bone turnover. Additionally, estrogens directly affects the structure and function of musculoskeletal tissues such as muscle, tendon, and ligament [
27,
28].
Undoubtedly, hypoestrogenism as well as nutritional deficiencies cause changes in bone turnover with inhibition of bone formation and an increase in resorption. After supplementing hormonal deficiencies, especially estrogens, a marked improvement in bone mineral density is observed in women with FHA [
29].
The timing and duration of amenorrhea is also important with respect to the amount of bone loss. In a prior study of 24 women with FHA compared to 31 normal, age-matched women, 83% were diagnosed with osteopenia [
30].
4.4. Psychological Effect
The concentration of all sex steroids, but most of all estrogens, significantly influences the mood and other psychological characteristics in patients with FHA. Estrogens modulate the activity of many neurotransmitters and neuromodulators in the brain, such as serotonin, noradrenaline, acetylcholine, and dopamine, thus regulating mood. The importance of stress and mental disorders in FHA may have a bidirectional effect. Both psychological stress can be one of the causes of FHA, and FHA can have a huge impact on the emotional state of a patient suffering from FHA. Women with FHA have been shown to have significantly higher depression scores, greater anxiety, and greater difficulty coping with daily stress in comparison to healthy people [
31]. Elevated cortisol levels may also be a contributing factor to increased stress in FHA patients, leading to many psychiatric symptoms including anxiety and depression.
Specific character profiles are also very specific to women with FHA. These include dysfunctional attitudes, as well as perfectionist behavior and paying more attention to the opinions of others compared to healthy women. Very often, patients with FHA show greater attention to their appearance, fear and fear of weight gain, which has a negative impact on the diet and mental state. Additionally, we observe lower self-esteem, insecurity, and a sense of being out of control of their own lives in FHA patients [
32].
In the publication of Giles et al. in
Fertility and Sterility, the authors assessed the correlation between cognitive function, emotional and psychiatric history among patients diagnosed with FHA in comparison with patients with amenorrhea for other reasons and with a group of healthy patients. In each of the women included in the study group, the basis of the amenorrhea was analyzed by completing structured psychiatric interviews and self-report questionnaires. Among others, perfectionism, concern for the judgments of others (dysfunctional attitudes), coping ability and interpersonal dependence were assessed. More dysfunctional attitudes and greater difficulties in coping with the daily stress were shown in the FHA patients compared to healthy women. Additionally, women with FHA were more likely to have a history of mental disorders, most notably mood disorders, than women with eumenorrheic disorders, but did not differ from women with organic amenorrhea [
33].
Unfortunately, there is also an increased mortality in women with FHA caused by low body weight. The increased mortality is due to numerous long-term serious consequences and is by far the greatest in women with eating disorders. Mortality in anorexia nervosa patients is almost 10%. The sudden deaths result from contractility disorders, which can be associated with decreased myocardial mass and resultant electrolyte imbalances. Moreover, girls with anorexia are at an increased risk of suicide attempts [
34].
5. Treatment
The most appropriate and effective treatment for FHA is that of treating the underlying cause of hypogonadotropic hypogonadism. It includes providing the right amount of energy in the form of sufficient calories, stopping excessive sports or reducing stress, or the ability to deal with it. Only when treatment is effective is it possible to prevent the many complications of FHA. Very often, anxiety and mood disorders coexist with FHA, therefore the help of a psychologist and psychiatrist should be taken into account. Hospital treatment is required in the event of cardiac arrhythmias, most often bradycardia, water and electrolyte disturbances, or in cases of extreme malnutrition associated with eating disorders.
First of all, the focus should be on weight gain in patients, which is a broadly understood lifestyle change. The recovery of FHA patients with low body weight is associated with an increase in BMI or fat mass. It is on the part of doctors to encourage patients to gain weight by changing their lifestyle in terms of increasing caloric intake and reducing exercise. The need for a proper, healthy diet should be explained, and it should be adjusted to the energy expenditure. In addition, it is important to try to reduce stress through the use of various types of cognitive-behavioral and family therapy [
6,
35].
Pharmacological treatment involving the use of replacement doses of hormones should be started after 6–12 months of ineffective non-pharmacological and behavioral lifestyle modifications. The most appropriate therapy that should be administered is transdermal cyclic estrogen-progestogen therapy. It can have a positive effect on cognitive functions, obsessive pursuit of a slim figure, and reduction of anxiety and dissatisfaction with one’s appearance in patients with FHA [
36].
In patients who want to become pregnant, also before starting pharmacological treatment, we initially suggest returning to normal body weight. Increasing the energy supply is a highly effective method in restoring normal estradiol levels and returning ovulatory cycles. Pharmacological treatment primarily consists of administering clomiphene citrate to induce ovulation. In the event of ineffectiveness, administration of exogenous gonadotropins and pulsatile GnRH administration (but not available in many countries) may be considered [
37,
38]. Regarding sexual function, some patients with FHA require topical estrogens to treat vaginal dryness and dyspareunia.
Due to the dangerous complication of FHA, which is the loss of bone mineral density, supplementing with vitamin D and calcium should be borne in mind. According to the recommendations of the Endocrine Society from 2017, the use of transdermal cyclic estrogen-progesterone therapy has an effect on bone protection and reduction of the risk of fractures in women with FHA. Unfortunately, treatment is often tedious and long-lasting, and until it brings encouraging results, patients withdraw from treatment and be discouraged. Additional research is needed to improve the treatment.
6. Conclusions
FHA is a common disorder that most commonly affects adolescents and reproductive women. The cause of the disorder is widely understood to be stress in various forms. Disorders caused by limiting caloric consumption are observed, causing weight loss, intensive sports by the patient and the occurrence of strong stressful experiences. The diagnosis of FHA should be made as soon as possible, taking into account a thorough medical history and analysis of the patient’s history, excluding other causes of amenorrhea. This is important, because providing the patient with immediate care and introducing pharmacological treatment at the right time avoids many harmful consequences that extend far beyond the reproductive system. Treatment is best carried out in cooperation with a gynecologist, endocrinologist, psychologist/psychiatrist and a dietitian. The goal of the future is to introduce new drugs that may prove to be more effective than the hormone replacement therapy used so far in most countries.
Author Contributions
Conceptualization, A.P. and B.M.; methodology, A.P.; software, A.P.; validation, A.P., B.M.; formal analysis, B.M.; investigation, A.P.; resources, A.P.; data curation, A.P. and B.M.; writing—original draft preparation, A.P.; writing—review and editing, A.P.; visualization, A.P.; supervision, B.M.; project administration, A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gordon, C.M. Clinical practice. Functional hypothalamic amenorrhea. N. Engl. J. Med. 2010, 363, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.S. Female hypogonadotropic hypogonadism. Hypothalamic amenorrhea syndrome. Endocrinol. Metab. Clin. N. Am. 1993, 22, 29–58. [Google Scholar] [CrossRef]
- Caronia, L.M.; Martin, C.; Welt, C.K.; Sykiotis, G.P.; Quinton, R.; Thambundit, A.; Avbelj, M.; Dhruvakumar, S.; Plummer, L.; Hughes, V.A.; et al. A genetic basis for functional hypothalamic amenorrhea. N. Engl. J. Med. 2011, 364, 215–225. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, E.; Goodman, J.M.; Mak, S.; Harvey, P.J. Impaired vascular function in physically active premenopausal women with functional hypothalamic amenorrhea is associated with low shear stress and increased vascular tone. J. Clin. Endocrinol. Metab. 2014, 99, 1798–1806. [Google Scholar] [CrossRef] [Green Version]
- Michopoulos, V.; Mancini, F.; Loucks, T.L.; Berga, S.L. Neuroendocrine recovery initiated by cognitive behavioral therapy in women with functional hypothalamic amenorrhea: A randomized, controlled trial. Fertil. Steril. 2013, 99, 2084–2091. [Google Scholar] [CrossRef] [Green Version]
- Roa, J.; Tena-Sempere, M. KiSS-1 system and reproduction: Comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen. Comp. Endocrinol. 2007, 153, 132–140. [Google Scholar] [CrossRef]
- Perkins, R.B.; Hall, J.E.; Martin, K.A. Neuroendocrine abnormalities in hypothalamic amenorrhea: Spectrum, stability, and response to neurotransmitter modulation. J. Clin. Endocrinol. Metab. 1999, 84, 1905. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P.; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867. [Google Scholar]
- Misra, M.; Miller, K.K.; Bjornson, J.; Hackman, A.; Aggarwal, A.; Chung, J.; Ott, M.; Herzog, D.B.; Johnson, M.L.; Klibanski, A. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J. Clin. Endocrinol. Metab. 2003, 88, 5615. [Google Scholar] [CrossRef]
- Edozien, L.C. Mind over matter: Psychological factors and the menstrual cycle. Curr. Opin. Obstet. Gynecol. 2006, 18, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Mortola, J.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef]
- Kondoh, Y.; Uemura, T.; Murase, M.; Yokoi, N.; Ishikawa, M.; Hirahara, F. A longitudinal study of disturbances of the hypothalamic-pituitary-adrenal axis in women with progestin-negative functional hypothalamic amenorrhea. Fertil. Steril. 2001, 76, 748–752. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Slusarz, K.; Guereca, G.; Pierce, L.; Slattery, M.; Mendes, N.; Herzog, D.B.; Misra, M. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christo, K.; Cord, J.; Mendes, N.; Miller, K.K.; Goldstein, M.A.; Klibanski, A.; Misra, M. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: A cross-sectional study. Clin. Endocrinol. (Oxf.) 2008, 69, 628. [Google Scholar] [CrossRef]
- Harber, V.J.; Petersen, S.R.; Chilibeck, P.D. Thyroid hormone concentrations and muscle metabolism in amenorrheic and eumenorrheic athletes. Can. J. Appl. Physiol. 1998, 23, 293. [Google Scholar] [CrossRef]
- Thralls, K.J.; Nichols, J.F.; Barrack, M.T.; Kern, M.; Rauh, M.J. Body Mass-Related Predictors of the Female Athlete Triad Among Adolescent Athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 17. [Google Scholar] [CrossRef]
- Warren, M.P.; Brooks-Gunn, J.; Fox, R.P.; Holderness, C.C.; Hyle, E.P.; Hamilton, W.G. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: A longitudinal study. J. Clin. Endocrinol. Metab. 2002, 87, 3162–3168. [Google Scholar] [CrossRef]
- De Souza, M.J.; Toombs, R.J.; Scheid, J.L.; O’Donnell, E.; West, S.L.; Williams, N.I. High prevalence of subtle and severe menstrual disturbances in exercising women: Confirmation using daily hormone measures. Hum. Reprod. 2010, 25, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleußner, E. The prevention, diagnosis and treatment of premature labor. Dtsch. Arztebl. Int. 2013, 110, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Veleva, Z.; Tiitinen, A.; Vilska, S.; Hydén-Granskog, C.; Tomás, C.; Martikainen, H.; Tapanainen, J.S. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum. Reprod. 2008, 23, 878–884. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Sex steroids, cardiovascular disease, and hypertension: Unanswered questions and some speculations. Hypertension 2005, 45, 170–174. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.; Goodman, J.M.; Harvey, P.J. Clinical review: Cardiovascular consequences of ovarian disruption: A focus on functional hypothalamic amenorrhea in physically active women. J. Clin. Endocrinol. Metab. 2011, 96, 3638. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.L.; Blondon, M.; Wiggins, K.L.; Harrington, L.B.; van Hylckama Vlieg, A.; Floyd, J.S.; Hwang, M.; Bis, J.C.; McKnight, B.; Rice, K.M.; et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern. Med. 2014, 174, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Solomon, C.G.; Hu, F.B.; Dunaif, A.; Rich-Edwards, J.E.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Menstrual Cycle Irregularity and Risk for Future Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2002, 87, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Nazem, T.; Chapko, D.; Russell, M.; Mendes, N.; Taylor, A.P.; Bouxsein, M.L.; Misra, M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J. Clin. Endocrinol. Metab. 2011, 96, 3123. [Google Scholar] [CrossRef]
- Christo, K.; Prabhakaran, R.; Lamparello, B.; Cord, J.; Miller, K.K.; Goldstein, M.A.; Gupta, N.; Herzog, D.B.; Klibanski, A.; Misra, M. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics 2008, 121, 1127. [Google Scholar] [CrossRef] [Green Version]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019, 9, 1834. [Google Scholar] [CrossRef]
- Misra, M.; Katzman, D.; Miller, K.K.; Mendes, N.; Snelgrove, D.; Russell, M.; Goldstein, M.A.; Ebrahimi, S.; Clauss, L.; Weigel, T.; et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J. Bone Miner. Res. 2011, 26, 2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podfigurna-Stopa, A.; Pludowski, P.; Jaworski, M.; Lorenc, R.; Genazzani, A.R.; Meczekalski, B. Skeletal status and body composition in young women with functional hypothalamic amenorrhea. Gynecol. Endocrinol. 2012, 28, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.D.; Loucks, T.L.; Berga, S.L. Psychological correlates of functional hypothalamic amenorrhea. Fertil. Steril. 2001, 76, 310–316. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, D.E.; Berga, S.L. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: A controlled comparison. Fertil. Steril. 1993, 60, 486–492. [Google Scholar] [CrossRef]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005, 293, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berga, S.L.; Marcus, M.D.; Loucks, T.L.; Hlastala, S.; Ringham, R.; Krohn, M.A. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil. Steril. 2003, 80, 976–981. [Google Scholar] [CrossRef]
- Baskaran, C.; Cunningham, B.; Plessow, F.; Singhal, V.; Woolley, R.; Ackerman, K.E.; Slattery, M.; Lee, H.; Lawson, E.A.; Eddy, K. Estrogen Replacement Improves Verbal Memory and Executive Control in Oligomenorrheic/Amenorrheic Athletes in a Randomized Controlled Trial. J. Clin. Psychiatry 2017, 78, e490–e497. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.E.; Morgante, G.; Musacchio, M.C.; Petraglia, F.; De Leo, V. New protocol of clomiphene citrate treatment in women with hypothalamic amenorrhea. Gynecol. Endocrinol. 2007, 23, 343. [Google Scholar] [CrossRef]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional hypothalamic amenorrhea and its influence on women’s health. J. Endocrinol. Investig. 2014, 37, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).