The Physiological Role of Irisin in the Regulation of Muscle Glucose Homeostasis
Abstract
:1. Introduction
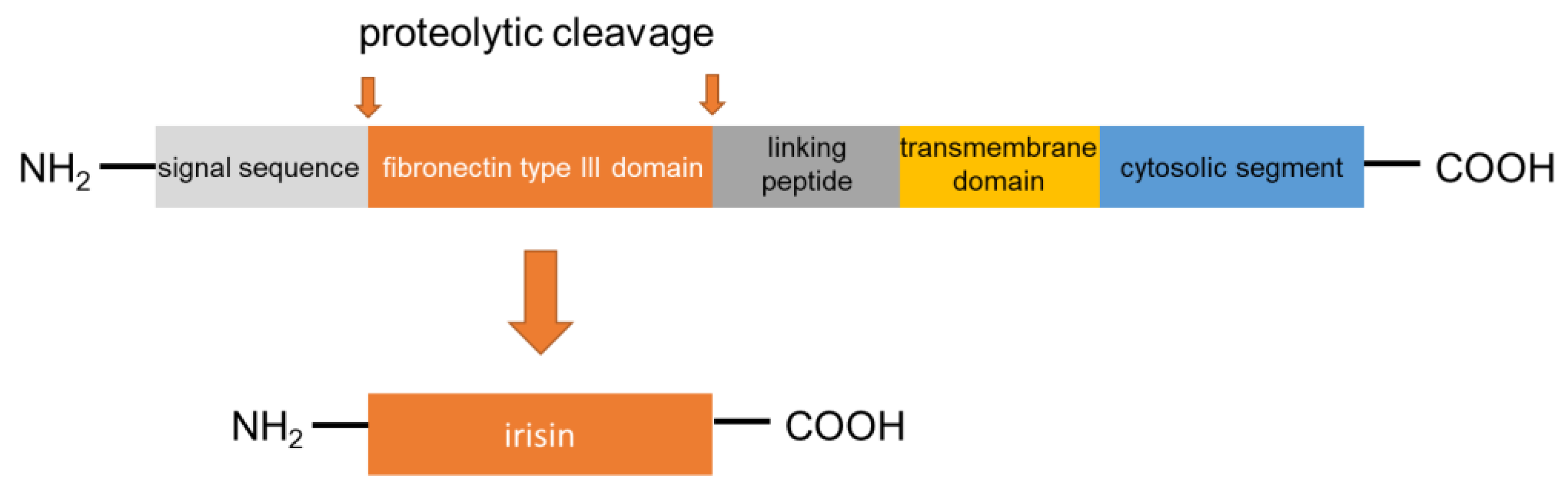
2. Synthesis and Secretion of Irisin
3. Muscle Glucose Homeostasis in Patients with Metabolic Diseases: A Role for Irisin
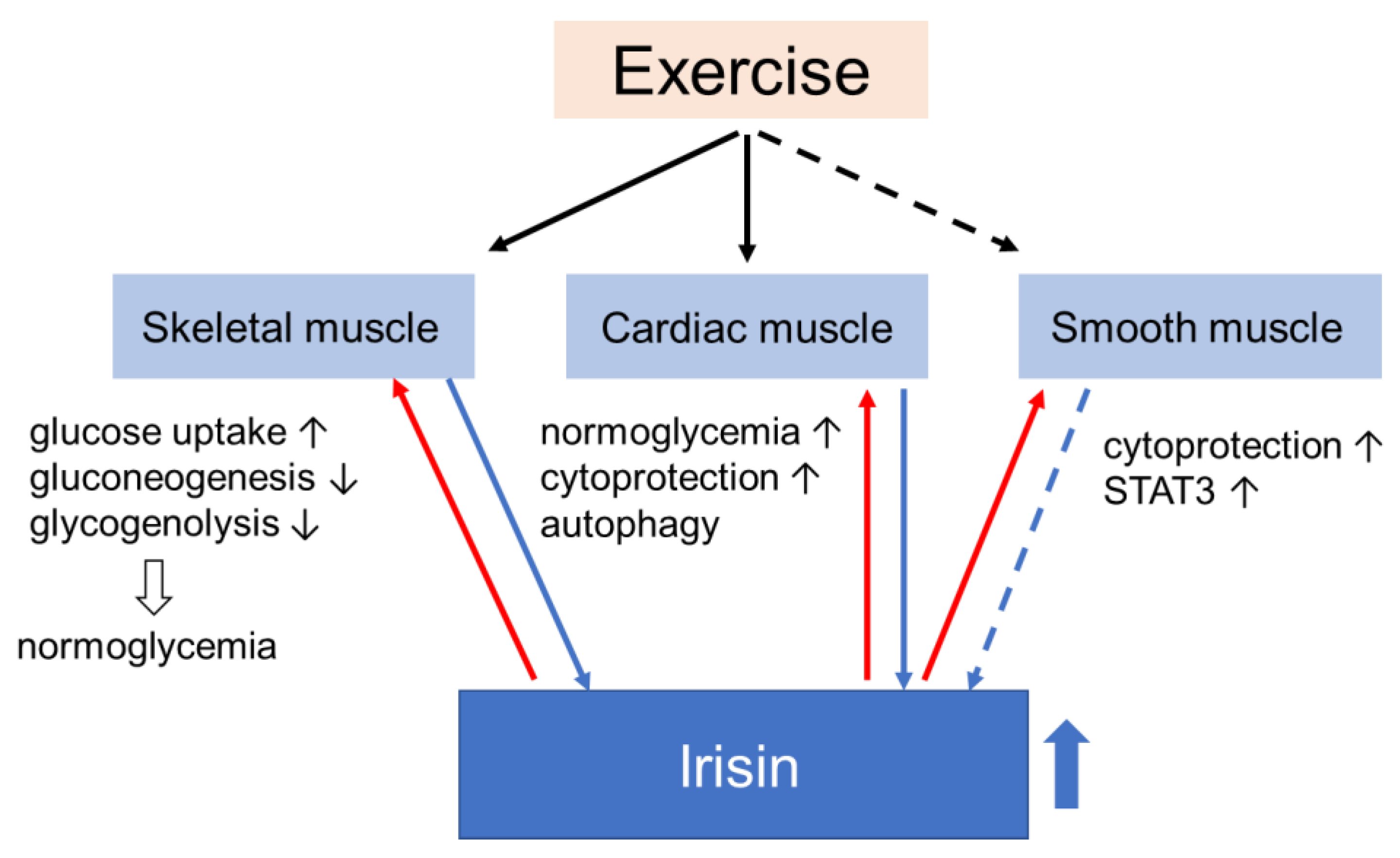
4. Effects of Irisin on Muscle Glucose Homeostasis
4.1. Skeletal Muscle
4.2. Smooth Muscle
4.3. Myocardium
4.4. Effects of Irisin on Mitochondria to Preserve Muscle Glucose Homeostasis
4.5. The Effects of Irisin on Systemic Glucose Homeostasis
4.5.1. Interactions of Irisin and Other Hormones
4.5.2. Interventional Animal Studies
4.5.3. Human Studies
4.6. Applicability of Irisin in the Treatment of Diabetic Complications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Sousa, R.A.L. Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals Diabetes mellitus. Int. J. Diabetes Dev. Ctries 2018, 38, 138–145. [Google Scholar] [CrossRef]
- Ross, L.M.; Slentz, C.A.; Zidek, A.M.; Huffman, K.M.; Shalaurova, I.; Otvos, J.D.; Connelly, M.A.; Kraus, V.B.; Bales, C.W.; Houmard, J.A.; et al. Effects of Amount, Intensity, and Mode of Exercise Training on Insulin Resistance and Type 2 Diabetes Risk in the STRRIDE Randomized Trials. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Shah, S.Z.A.; Karam, J.A.; Zeb, A.; Ullah, R.; Shah, A.; Haq, I.U.; Ali, I.; Darain, H.; Chen, H. Movement is improvement: The therapeutic effects of exercise and general physical activity on glycemic control in patients with type 2 diabetes mellitus: A systematic re-view and meta-analysis of randomized controlled trials. Diabetes Ther. 2021, 12, 707–732. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Devage, S.; Constantine, G.R.; Katulanda, P.; Hills, A.P.; King, N.A. Glycemic and cardiometabolic effects of exercise in South Asian Sri Lankans with type 2 diabetes mellitus: A randomized controlled trial Sri Lanka diabetes aerobic and re-sistance training study (SL-DARTS). Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.; Hills, A.P.; Constantine, G.R.; Finlayson, G.; Katulanda, P.; King, N.A. Study protocol: A randomised controlled trial of supervised resistance training versus aerobic training in Sri Lankan adults with type 2 diabetes mellitus: SL-DART study. BMC Public Health 2018, 18, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Swardfager, W.; Fernandes, D.; Laredo, S.; Tomlinson, G.; Oh, P.I.; Thomas, S. Finding the optimal volume and intensity of resistance training exercise for type 2 diabetes: The FORTE Study, a randomized trial. Diabetes Res. Clin. Pract. 2017, 130, 98–107. [Google Scholar] [CrossRef]
- Crescioli, C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin, D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.G.; Lopes, M.A.; Batista, M.L.J. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Pedersen, A.J.T.; Kristensen, J.M.; Handberg, A.; Wojtaszewski, J.F.P.; Højlund, K. Intact regulation of muscle expres-sion and circulating levels of myokines in response to exercise in patients with type 2 diabetes. Physiol. Rep. 2018, 6, e13723. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Kaspric, N.; Vonnahme, K.; Viala, D.; Chambon, C.; Picard, B. Prediction of the secretome and the surfaceome: A strat-egy to decipher the crosstalk between adipose tissue and muscle during fetal growth. Int. J. Mol. Sci. 2020, 21, 4375. [Google Scholar] [CrossRef]
- White, P.J.; St-Pierre, P.; Charbonneau, A.; Mitchell, P.L.; St-Amand, E.; Marcotte, B.; Marette, A. Protectin DX alleviates insulin re-sistance by activating a myokine-liver glucoregulatory axis. Nat. Med. 2014, 20, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Jodeiri Farshbaf, M.; Garasia, S.; Moussoki, D.P.K.; Mondal, A.K.; Cherkowsky, D.; Manal, N.; Alviña, K. Hippocampal injection of the exercise-induced myokine irisin suppresses acute stress-induced neurobehavioral impairment in a sex-dependent manner. Behav. Neurosci. 2020, 134, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Ciaraldi, T.P.; Henry, R.R. Myokine Regulation of Insulin Secretion: Impact of Inflammation and Type 2 Diabetes. Front. Physiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Mahajan, R.D.; Patra, S.K. Irisin, a Novel Myokine Responsible for Exercise Induced Browning of White Adipose Tissue. Indian J. Clin. Biochem. 2013, 28, 102–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2013, 281, 739–749. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The myokine irisin is released in response to saturated fatty acids and promotes pancreatic β-cell sur-vival and insulin secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes/Metab. Res. Rev. 2016, 32, 51–59. [Google Scholar] [CrossRef]
- Leung, P.S. The potential of irisin as a therapeutic for diabetes. Future Med. Chem. 2017, 9, 529–532. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Fibronectin Type III Domain-Containing Protein 5 Precursor [Rattus Norvegicus]. 2016. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/protein/NP_001257910.1 (accessed on 1 December 2020).
- NCBI. Fibronectin Type III Domain-Containing Protein 5 Preproprotein [Mus Musculus]. 2016. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/protein/NP_081678.1 (accessed on 1 December 2020).
- NCBI. Fibronectin Type III Domain-Containing Protein 5 Isoform 2 Preproprotein [Homo Sapiens]. 2016. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/protein/NP_715637.2 (accessed on 1 December 2020).
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [Green Version]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodríguez, B.M.; Pena-Bello, L.; Juiz-Valiña, P.; Vidal-Bretal, B.; Cordido, F.; Sangiao-Alvarellos, S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 2016, 6, 29898. [Google Scholar] [CrossRef] [Green Version]
- Gür, F.M.; Timurkaan, S.; Yalcin, M.H.; Girgin, A.; Tarakçı, B.G. Immunohistochemical localization of irisin in mole rats(Spalax leucodon). Biotech. Histochem. 2017, 92, 245–251. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Cheng, Y.; Zhao, L.; Chen, Y.; Liu, Y. The relationships of irisin with bone mineral density and body composition in PCOS patients. Diabetes/Metab. Res. Rev. 2016, 32, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ke, Y.; Wu, F.; Liu, S.; Ji, C.; Zhu, X.; Zhang, Y. Circulating irisin levels in patients with nonalcoholic fatty liver disease: A sys-tematic review and meta-analysis. Gastroenterol. Res. Pract. 2020, 2020. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Perez-Quilis, C. The p38-PGC-1α-irisin-betatrophin axis: Exploring new pathways in insulin resistance. Adipocyte 2014, 3, 67–68. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.-Q.; Chen, D.; Sun, H.-J.; Ding, L.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Zhou, Y.-B.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Quan, J.I. From white to brown fat through the PGC-1α-dependent myokine irisin: Implications for diabetes and obe-sity. Dis. Model. Mech. 2012, 5, 293–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciorkowska, M.; Musiałowska, D.; Małyszko, J. Adropin and irisin in arterial hypertension, diabetes mellitus and chronic kidney disease. Adv. Clin. Exp. Med. 2019, 28, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In Vivo and In Vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Holten, M.K.; Zacho, M.; Gaster, M.; Juel, C.; Wojtaszewski, J.; Dela, F. Strength training increases insulin-mediated glucose uptake, GLUT4 content and insulin signaling in skeletal muscle inpatients with Type 2 diabetes. Diabetes 2004, 53, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wen, L.; Zhou, S.; Zhang, Y.; Wang, X.H.; He, Y.Y.; Davie, A.; Broadbent, S. Effects of four weeks intermittent hypoxia in-tervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and Akt activ-ity in skeletal muscle of obese mice with type 2 diabetes. PLoS ONE 2018, 13, e0203551. [Google Scholar]
- Dela, F.; Ingersen, A.; Andersen, N.B.; Nielsen, M.B.; Petersen, H.H.H.; Hansen, C.N.; Larsen, S.; Wojtaszewski, J.; Helge, J.W. Effects of one-legged high-intensity interval training on insulin-mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol. 2019, 226, e13245. [Google Scholar] [CrossRef]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kir wan, J.P. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef] [Green Version]
- Glynn, N.W.; Gmelin, T.; Santanasto, A.; Lovato, L.C.; Lange-Maia, B.S.; Nicklas, B.J.; Fielding, R.A.; Manini, T.M.; Myers, V.H.; de Rekeneire, N.; et al. Impact of Baseline Fatigue on a Physical Activity Intervention to Prevent Mobility Disability. J. Am. Geriatr. Soc. 2020, 68, 619–624. [Google Scholar] [CrossRef]
- Bone, D.B.J.; Meister, J.; Knudsen, J.R.; Dattaroy, D.; Cohen, A.; Lee, R.; Lu, H.; Metzger, D.; Jensen, T.E.; Wess, J. Skeletal muscle-specific activation of Gq signaling maintains glucose homeostasis. Diabetes 2019, 68, 1341–1352. [Google Scholar] [CrossRef]
- Jia, R.; Li, Z.; Ou, Z.; Wu, J.; Sun, B.; Lin, L.; Zhao, M. Physicochemical Characterization of Hizikia fusiforme Polysaccharide and Its Hypoglycemic Activity via Mediating Insulin-Stimulated Blood Glucose Utilization of Skeletal Muscle in Type 2 Diabetic Rats. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef]
- Acheson, K.J.; Schutz, Y.; Bessard, T.; Anantharaman, K.; Flatt, J.P.; Jéquier, E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am. J. Clin. Nutr. 1988, 48, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komosinska-Vassev, K.; Gala, O.; Olczyk, K.; Jura-Półtorak, A.; Olczyk, P. The usefulness of diagnostic panels based on circulating adipocytokines/regulatory peptides, renal function tests, insulin resistance indicators and lipid-carbohydrate metabolism pa-rameters in diagnosis and prognosis of type 2 diabetes mellitus with obesity. Biomolecules 2020, 10, 1304. [Google Scholar]
- Wall, V.Z.; Barnhart, S.; Kanter, J.E.; Kramer, F.; Shimizu-Albergine, M.; Adhikari, N.; Wight, T.N.; Hall, J.L.; Bornfeldt, K.E. Smooth muscle glucose metabolism promotes monocyte recruitment and atherosclerosis in a mouse model of metabolic syndrome. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Katoh, C.; Beanlands, R.S.; Noriyasu, K.; Komuro, K.; Yamada, S.; Kuge, Y.; Morita, K.; Kitabatake, A.; Tamaki, N. Reduced oxidative metabolic response in dysfunctional myocardium with preserved glucose metabolism but with impaired contractile reserve. J. Nucl. Med. 2004, 45, 1885–1891. [Google Scholar]
- Zhang, D.; Xie, T.; Leung, P.S. Irisin ameliorates glucolipotoxicity-associated β-cell dysfunction and apoptosis via AMPK signal-ing and anti-inflammatory actions. Cell Physiol. Biochem. 2018, 51, 924–937. [Google Scholar] [CrossRef]
- Liu, Y.; Chewchuk, S.; Lavigne, C.; Brûlé, S.; Pilon, G.; Houde, V.; Xu, A.; Marette, A.; Sweeney, G. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am. J. Physiol. Metab. 2009, 297, E657–E664. [Google Scholar] [CrossRef]
- Ellefsen, S.; Vikmoen, O.; Slettaløkken, G.; Whist, J.E.; Nygaard, H.; Hollan, I.; Rauk, I.; Vegge, G.; Strand, T.A.; Raastad, T.; et al. Irisin and FNDC5: Effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur. J. Appl. Physiol. 2014, 114, 1875–1888. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6490–6497. [Google Scholar]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle me-tabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [Green Version]
- Yano, N.; Zhang, L.; Wei, D.; Dubielecka, P.M.; Wei, L.; Zhuang, S.; Zhu, P.; Qin, G.; Liu, P.Y.; Chin, Y.E.; et al. Irisin counteracts high glucose and fatty acid-induced cytotoxicity by preserving the AMPK-insulin receptor signaling axis in C2C12 myoblasts. Am. J. Physiol. Metab. 2020, 318, E791–E805. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; Zhu, H.; Xu, J.; Yang, L.; Liu, L.; Li, J. β-arrestin-2 is involved in irisin induced glucose metabolism in type 2 diabetes via p38 MAPK signaling. Exp. Cell Res. 2017, 360, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shen, Y.; Ni, C.; Ye, J.; Xin, Y.; Zhang, W.; Ren, Y. Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1α pathway. Peptides 2019, 119, 170120. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Liu, J.; Zhang, J.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Ye, J.; Lian, K.; Xu, C.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef]
- Kobashigawa, L.C.; Xu, Y.C.; Padbury, J.F.; Tseng, Y.T.; Yano, N. Metformin protects cardiomyocyte from doxorubicin induced cy-totoxicity through an AMP-activated protein kinase dependent signaling pathway: An in vitro study. PLoS ONE 2014, 9, e104888. [Google Scholar] [CrossRef] [Green Version]
- Klip, A.; Leiter, L.A. Cellular Mechanism of Action of Metformin. Diabetes Care 1990, 13, 696–704. [Google Scholar] [CrossRef]
- Cusi, K.; Consoli, A.; DeFronzo, R.A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067. [Google Scholar] [CrossRef] [Green Version]
- Turban, S.; Stretton, C.; Drouin, O.; Green, C.; Watson, M.L.; Gray, A.; Ross, F.; Lantier, L.; Viollet, B.; Hardie, G.; et al. Defining the Contribution of AMP-activated Protein Kinase (AMPK) and Protein Kinase C (PKC) in Regulation of Glucose Uptake by Metformin in Skeletal Muscle Cells. J. Biol. Chem. 2012, 287, 20088–20099. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-T.; Chen, W.; Chung, H.-H.; Cheng, K.-C.; Yeh, C.-H.; Cheng, J.-T. Activation of Imidazoline I-2B Receptor by Metformin to Increase Glucose Uptake in Skeletal Muscle. Horm. Metab. Res. 2011, 43, 708–713. [Google Scholar] [CrossRef]
- Li, D.-J.; Huang, F.; Lu, W.-J.; Jiang, G.-J.; Deng, Y.-P.; Shen, F.-M. Metformin promotes irisin release from murine skeletal muscle in-dependently of AMP-activated protein kinase activation. Acta Physiol. 2015, 213, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Mayer, A.; Maderova, D.; Belan, V.; Wolfrum, C.; Ukropec, J.; Ukropcova, B. Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes. Peptides 2014, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is ex-pressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endo-crinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Song, H.; Xu, J.; Lv, N.; Zhang, Y.; Wu, F.; Li, H.; Shao, L.; Mu, Q.; Wang, F.; Tang, N.; et al. Irisin reverses platelet derived growth factor-BB-induced vascular smooth muscle cells phenotype modulation through STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2016, 479, 139–145. [Google Scholar] [CrossRef]
- Cho, S.J.; Moon, J.S.; Lee, C.M.; Choi, A.M.; Stout-Delgado, H.W. Glucose transporter 1-dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 521–531. [Google Scholar] [CrossRef]
- Li, M.; Jia, F.; Zhou, H.; Di, J.; Yang, M. Elevated aerobic glycolysis in renal tubular epithelial cells influences the proliferation and differentiation of podocytes and promotes renal interstitial fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5082–5090. [Google Scholar] [PubMed]
- Khodarahmi, A.; Eshaghian, A.; Safari, F.; Moradi, A. Quercetin mitigates hepatic insulin resistance in rats with bile duct ligation through modulation of the STAT3/SOCS3/IRS1 signaling pathway. J. Food Sci. 2019, 84, 3045–3053. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, Y.; Tweardy, D.J.; Mitch, W.E. Stat3 activation induces insulin resistance via a muscle-specific E3 ubiq-uitin ligase Fbxo40. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E625–E635. [Google Scholar] [CrossRef]
- Song, H.-T.; Cui, Y.; Zhang, L.-L.; Cao, G.; Li, L.; Li, G.; Jia, X.-J. Ruxolitinib attenuates intimal hyperplasia via inhibiting JAK2/STAT3 signaling pathway activation induced by PDGF-BB in vascular smooth muscle cells. Microvasc. Res. 2020, 132, 104060. [Google Scholar] [CrossRef]
- White, A.T.; LaBarge, S.A.; McCurdy, C.E.; Schenk, S. Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance. Mol. Metab. 2015, 4, 569–575. [Google Scholar] [CrossRef]
- Jamshidi, Y.; Kyriakou, T.; Gooljar, S.B.; Collins, L.J.; Lane, C.A.; Snieder, H.; Wang, X.; Spector, T.D.; O’Dell, S.D. Common STAT3 vari-ants are not associated with obesity or insulin resistance in female twins. Obesity 2007, 15, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Gianotti, T.F.; Sookoian, S.; Gemma, C.; Burgueño, A.L.; Gonzalez, C.; Pirola, C.J. Study of Genetic Variation in the STAT3 on Obesity and Insulin Resistance in Male Adults. Obesity 2008, 16, 1702–1707. [Google Scholar] [CrossRef]
- Osman, I.; Segar, L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem. Pharmacol. 2016, 101, 54–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togliatto, G.; Dentelli, P.; Rosso, A.; Lombardo, G.; Gili, M.; Gallo, S.; Gai, C.; Solini, A.; Camussi, G.; Brizzi, M.F. PDGF-BB Carried by Endothelial Cell–Derived Extracellular Vesicles Reduces Vascular Smooth Muscle Cell Apoptosis in Diabetes. Diabetes 2018, 67, 704–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Mummidi, S.; Sukhanov, S.; Yoshida, T.; Noda, M.; Delafontaine, P.; Chandrasekar, B. Minocycline inhibits PDGF-BB-induced human aortic smooth muscle cell proliferation and migration by reversing miR-221- and -222-mediated RECK suppression. Cell. Signal. 2019, 57, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, İ.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Gamble, J. Acetyl-CoA carboxylase: An important regulator of fatty acid oxidation in the heart. Can. J. Physiol. Pharmacol. 1994, 72, 1101–1109. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Viglino, C.; Brulhart-Meynet, M.-C.; James, R.; Lerch, R.; Montessuit, C. Impaired stimulation of glucose transport in cardiac myocytes exposed to very low-density lipoproteins. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhu, Q.; Wu, Z.; Ding, J.; Qin, S.; Liu, H.; Miao, P. Protective effects of irisin on hypoxia-reoxygenation injury in hyperglyce-mia-treated cardiomyocytes: Role of AMPK pathway and mitochondrial protection. J. Cell. Physiol. 2020, 235, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.T.; Zhang, L.; Dubielecka, P.M.; Zhuang, S.; Qin, G.; Chin, Y.E.; Zhang, S.; Zhao, T.C. Irisin improves myocardial per-formance and attenuates insulin resistance in spontaneous mutation (Lepr db) mice. Front. Pharmacol. 2020, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhao, X.; Cao, R.; Liang, Y.; Zhang, D.-Q.; Wang, R. Irisin improves insulin resistance by inhibiting autophagy through the PI3K/Akt pathway in H9c2 cells. Gene 2021, 769, 145209. [Google Scholar] [CrossRef]
- Kitada, M.; Takeda, A.; Nagai, T.; Ito, H.; Kanasaki, K.; Koya, D. Dietary restriction ameliorates diabetic nephropathy through an-ti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: A model of type 2 diabetes. Exp. Diabetes Res. 2011, 2011, 908185. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Tian, Z.; Sun, Y.; Lu, C.; Liu, N.; Gao, Z.; Zhang, L.; Dong, S.; Yang, F.; Zhong, X.; et al. Exogenous H 2 S facili-tating ubiquitin aggregates clearance via autophagy attenuates type 2 diabetes-induced cardiomyopathy. Cell Death Dis. 2017, 8, e2992. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Rijal, B.; Xu, M.; Li, Z.; An, Y.; Zhang, F.; Lu, C. Renal denervation improves vascular endothelial dysfunction by inducing autophagy via AMPK/mTOR signaling activation in a rat model of type 2 diabetes mellitus with insulin resistance. Acta Diabetol. 2020, 57, 1–17. [Google Scholar] [CrossRef]
- Ohsumi, Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001, 2, 211–216. [Google Scholar] [CrossRef]
- Zois, C.E.; Koukourakis, M.I. Radiation-induced autophagy in normal and cancer cells: Towards novel cytoprotection and ra-dio-sensitization policies? Autophagy 2009, 5, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Zhou, Y.; Liang, G.; Yang, B.; Yang, M.; King, A.; Wei, H. General Anesthetics Regulate Autophagy via Modulating the Inositol 1,4,5-Trisphosphate Receptor: Implications for Dual Effects of Cytoprotection and Cytotoxicity. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial in-farction. Aging 2020, 12, 4474–4488. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin promotes osteogenic differentiation of bone marrow mesenchymal stem cells by activating autophagy via the Wnt//β-catenin signal pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef]
- Mellor, K.M.; Bell, J.R.; Young, M.J.; Ritchie, R.H.; Delbridge, L.M. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J. Mol. Cell. Cardiol. 2011, 50, 1035–1043. [Google Scholar] [CrossRef]
- Xu, X.; Ren, J. Unmasking the janus faces of autophagy in obesity-associated insulin resistance and cardiac dysfunction. Clin. Exp. Pharmacol. Physiol. 2012, 39, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, D.; Yuan, J.; Yan, L.; Zhan, Z.; Pan, D.; Lin, L.; Mu, B. Compound danshen dripping pills prevented leptin deficien-cy-induced hepatic ER stress, stimulated autophagy, and improved insulin resistance of ob/ob mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 5368657. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.; Hotamisligil, G.S. Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ost, A.; Svensson, K.; Ruishalme, I.; Brännmark, C.; Franck, N.; Krook, H.; Sandström, P.; Kjolhede, P.; Strålfors, P. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol. Med. 2010, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Morales-Scholz, M.G.; Swinton, C.; Murphy, R.M.; Kowalski, G.M.; Bruce, C.R.; Howlett, K.F.; Shaw, C.S. Autophagy is not involved in lipid accumulation and the development of insulin resistance in skeletal muscle. Biochem. Biophys. Res. Commun. 2021, 534, 533–539. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Yang, D.; Yu, X.; Irwin, D.M.; Niu, G.; Tan, H. Excessive Autophagy Activation and Increased Apoptosis Are Associated with Palmitic Acid-Induced Cardiomyocyte Insulin Resistance. J. Diabetes Res. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and Autophagy: First Update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Piquereau, J.; Veksler, V.; Garnier, A. Estrogens, Estrogen Receptors Effects on Cardiac and Skeletal Muscle Mitochondria. Front. Endocrinol. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, G.S.; Simoes, E.; Lima, J.D.C.C.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M. Human cachexia induces changes in mitochondria, autophagy and apoptosis in the skeletal muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, S.; Kim, S.-J.; Koo, Y.D.; Lee, J.H.; Kim, H.; Ahn, B.; Ha, Y.-C.; Kim, Y.-H.; Jang, M.G.; Koo, K.-H.; et al. A mitochondrial proteome profile indicative of type 2 diabetes mellitus in skeletal muscles. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappler, L.; Kollipara, L.; Lehmann, R.; Sickmann, A. Investigating the role of mitochondria in type 2 diabetes—Lessons from lip-idomics and proteomics studies of skeletal muscle and liver. Adv. Exp. Med. Biol. 2019, 1158, 143–182. [Google Scholar]
- Xu, D.; Jiang, Z.; Sun, Z.; Wang, L.; Zhao, G.; Hassan, H.M.; Fan, S.; Zhou, W.; Han, S.; Zhang, L.; et al. Mitochondrial dysfunction and inhibition of myoblast differentiation in mice with high-fat-diet-induced pre-diabetes. J. Cell. Physiol. 2019, 234, 7510–7523. [Google Scholar] [CrossRef]
- Dohl, J.; Foldi, J.; Heller, J.; Gasier, H.G.; Deuster, P.A.; Yu, T. Acclimation of C2C12 myoblasts to physiological glucose concentra-tions for in vitro diabetes research. Life Sci. 2018, 211, 238–244. [Google Scholar] [CrossRef]
- Sarparanta, J.; Garcia-Macia, M.; Singh, R. Autophagy and Mitochondria in Obesity and Type 2 Diabetes. Curr. Diabetes Rev. 2017, 13, 352–369. [Google Scholar] [CrossRef]
- Lai, N.; Kummitha, C.; Hoppel, C. Defects in skeletal muscle subsarcolemmal mitochondria in a non-obese model of type 2 dia-betes mellitus. PLoS ONE 2017, 12, e0183978. [Google Scholar] [CrossRef] [Green Version]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, P.; Chen, Q.; Li, C. Exercise enhances mitochondrial fission and mitophagy to improve myopathy following criti-cal limb ischemia in elderly mice via the PGC1a/FNDC5/irisin pathway. Skelet. Muscle 2020, 10, 25. [Google Scholar] [CrossRef]
- Chen, K.; Xu, Z.; Liu, Y.; Wang, Z.; Li, Y.; Xu, X.; Chen, C.; Xia, T.; Liao, Q.; Yao, Y.; et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017, 9, eaao6298. [Google Scholar] [CrossRef] [Green Version]
- Srinivasa, S.; Suresh, C.; Mottla, J.; Hamarneh, S.R.; Irazoqui, J.E.; Frontera, W.; Torriani, M.; Stanley, T.; Makimura, M. FNDC5 relates to skeletal muscle IGF-I and mitochondrial function and gene expression in obese men with reduced growth hormone. Growth Horm. IGF Res. 2016, 26, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Adamska, A.; Łebkowska, A.; Jacewicz, M.; Krentowska, A.; Hryniewicka, J.; Wołczyński, S.; Górska, M.; Kowalska, I. Serum concen-trations of betatrophin and its association with indirect indices of insulin resistance and beta cell function in women with polycystic ovary syndrome. Int. J. Endocrinol. 2017, 2017, 2316986. [Google Scholar] [CrossRef]
- Zhang, R.; Abou-Samra, A.B. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: Consensus and controversy. Cardiovasc. Diabetol. 2014, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Repiso, C.; Garcia-Serrano, S.; Rodriguez-Pacheco, F.; García-Escobar, E.; Haro-Mora, J.J.; Garcia-Arnes, J.; Valdés, S.; Gonzalo, M.; Soriguer, F.; Moreno-Ruiz, F.J.; et al. FNDC5 could be regulated by leptin in adipose tissue. Eur. J. Clin. Investig. 2014, 44, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Moreno, N.R.; Balaguer, I.; Méndez-Giménez, L.; Becerril, S.; Catalan, V.; Gomez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanaki, M.; Moradi, N.; Emamgholipour, S.; Fadaei, R.; Poustchi, H. Lower circulating irisin is associated with nonalcoholic fatty liver disease and type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S467–S472. [Google Scholar] [CrossRef]
- Shirvani, H.; Rahmati-Ahmadabad, S.; Broom, D.R.; Mirnejad, R. Eccentric resistance training and β-hydroxy-β-methylbutyrate free acid affects muscle PGC-1α expression and serum irisin, nesfatin-1 and resistin in rats. J. Exp. Biol. 2019, 222, jeb198424. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.B.; Kim, H.-J.; Kang, J.H.; Park, S.I.; Park, K.H.; Lee, H.-J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism 2017, 73, 100–108. [Google Scholar] [CrossRef]
- Çatlı, G.; Küme, T.; Tuhan, H.Ü.; Anık, A.; Çalan, Ö.G.; Böber, E.; Abacı, A. Relation of serum irisin level with metabolic and antro-pometric parameters in obese children. J. Diabetes Complic. 2016, 30, 1560–1565. [Google Scholar] [CrossRef]
- Blüher, S.; Panagiotou, G.; Petroff, D.; Markert, J.; Wagner, A.; Klemm, T.; Filippaios, A.; Keller, A.; Mantzoros, C.S. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity 2014, 22, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Becerril, S.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin admin-istration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int. J. Obes. 2015, 39, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Philippou, A.; Maridaki, M.; Tenta, R.; Koutsilieris, M. Hormonal responses following eccentric exercise in humans. Hormones 2017, 16, 405–413. [Google Scholar]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity—Correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [Green Version]
- Belviranli, M.; Okudan, N.; Çelik, F. Association of Circulating Irisin with Insulin Resistance and Oxidative Stress in Obese Women. Horm. Metab. Res. 2016, 48, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Marrano, N.; Biondi, G.; Borrelli, A.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Irisin and Incretin Hormones: Similarities, Differences, and Implications in Type 2 Diabetes and Obesity. Biomolecules 2021, 11, 286. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Farrash, W.; Brook, M.; Crossland, H.; Phillips, B.E.; Cegielski, J.; Wilkinson, D.J.; Constantin-Teodosiu, D.; Greenhaff, P.L.; Smith, K.; Cleasby, M.; et al. Impacts of rat hindlimb Fndc5/irisin overexpression on muscle and adipose tissue metabolism. Am. J. Physiol. Metab. 2020, 318, E943–E955. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Dalamaga, M.; Kim, S.Y.; Polyzos, S.A.; Hamnvik, O.P.; Magkos, F.; Paruthi, J.; Mantzoros, C.S. Leptin’s role in lipo-dystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr. Rev. 2013, 34, 377–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, X.; Lin, J.; Zhang, Y.; Zhou, L.; Xu, L.; Jia, J.; Zhao, B.; Lin, Z.; Zhu, Q.; Li, L.; et al. Serum Irisin Levels and Clinical Implication in Elderly Patients with Type 2 Diabetes Mellitus. J. Clin. Med. Res. 2020, 12, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Gonzalez-Gil, A.M.; Villarreal-Calderón, J.R.; Tamez-Rivera, O.; Rodriguez-Gutierrez, N.A.; Castillo, E.C.; Silva-Platas, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Serum irisin levels, endothelial dysfunction, and inflammation in pediatric patients with type 2 diabetes mellitus and metabolic syndrome. J. Diabetes Res. 2020, 2020, 1949415. [Google Scholar] [CrossRef]
- Huh, J.H.; Ahn, S.V.; Choi, J.H.; Koh, S.B.; Chung, C.H. High serum irisin level as an independent predictor of diabetes mellitus: A longitudinal population-based study. Medicine 2016, 95, e3742. [Google Scholar] [CrossRef]
- Bastu, E.; Zeybek, U.; Gurel, E.G.; Ozgor, B.Y.; Celik, F.; Okumus, N.; Demiral, I.; Dural, O.; Celik, C.; Bulut, H.; et al. Effects of Irisin and Exercise on Metabolic Parameters and Reproductive Hormone Levels in High-Fat Diet-Induced Obese Female Mice. Reprod Sci. 2018, 25, 281–291. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Zulet, M.A.; Lopez-Legarrea, P.; de la Iglesia, R.; Pardo, M.; Carreira, M.C.; Martínez, J.A.; Casanueva, F.F. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism 2014, 63, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Palacios-González, B.; Vadillo-Ortega, F.; Polo-Oteyza, E.; Sánchez, T.; Ancira-Moreno, M.; Romero-Hidalgo, S.; Meráz, N.; Antuna-Puente, B. Irisin levels before and after physical activity among school-age children with different BMI: A direct relation with leptin. Obesity 2015, 23, 729–732. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kurose, S.; Shinno, H.; Thu, H.C.T.; Takao, N.; Tsutsumi, H.; Hasegawa, T.; Nakajima, T.; Kimura, Y. Effects of Body Weight Reduction on Serum Irisin and Metabolic Parameters in Obese Subjects. Diabetes Metab. J. 2016, 40, 386–395. [Google Scholar] [CrossRef]
- Rashid, F.A.; Abbas, H.J.; Naser, N.A.; Addai Ali, H. Effect of Long-Term Moderate Physical Exercise on Irisin between Normal Weight and Obese Men. Sci. World J. 2020, 2020. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef]
- Most, R.S.; Sinnock, P. The Epidemiology of Lower Extremity Amputations in Diabetic Individuals. Diabetes Care 1983, 6, 87–91. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Sawada, N.; Jiang, A.; Takizawa, F.; Safdar, A.; Manika, A.; Tesmenitsky, Y.; Kang, K.-T.; Bischoff, J.; Kalwa, H.; Sartoretto, J.L.; et al. Endothelial PGC-1α Mediates Vascular Dysfunction in Diabetes. Cell Metab. 2014, 19, 246–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Q.; Qu, S.; Tang, L.-X.; Li, L.-P.; He, D.-F.; Zeng, C.Y.; Wang, W.E. Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis. Acta Pharmacol. Sin. 2019, 40, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, H.; Zhang, J.; Zhang, X.; Xin, C.; Zhang, F.; Lee, Y.; Zhang, L.; Lian, K.; Yan, W.; et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J. Mol. Cell. Cardiol. 2015, 87, 138–147. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, D.-I.; Choi, J.-H.; Heo, Y.-R.; Park, S.-H. New role of irisin in hepatocytes: The protective effect of hepatic steatosis in vitro. Cell. Signal. 2015, 27, 1831–1839. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | PMID * | Ref #** | ||
|---|---|---|---|---|---|
| 1. Animal studies | |||||
| 1. | A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis | Boström et al. | 2012 | 22237023 | [16] |
| 2. | The myokine irisin is released in response to saturated fatty acids and promotes pancreatic β-cell survival and insulin secretion | Natalicchio, et al. | 2017 | 28724742 | [20] |
| 3. | FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity | Xiong, et al. | 2015 | 26111885 | [34] |
| 4. | Irisin ameliorates glucolipotoxicity-associated β-cell dysfunction and apoptosis via AMPK signaling and anti-inflammatory actions | Zhang, et al. | 2018 | 30466091 | [50] |
| 5. | Decreased irisin secretion contributes to muscle insulin resistance in high fat diet mice | Yang, et al. | 2015 | 26261526 | [54] |
| 6. | Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy | Maisha Reza, et al. | 2017 | 29062100 | [134] |
| 7. | Impacts of rat hindlimb Fndc5/irisin overexpression on muscle and adipose tissue metabolism | Farrash, et al. | 2020 | 32369414 | [135] |
| 8. | Effects of irisin and exercise on metabolic parameters and reproductive hormone levels in high-fat diet-induced obese female mice | Bastu, et al. | 2018 | 28594316 | [141] |
| 2. Human studies | |||||
| 1. | Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies | Kurdiova, et al. | 2014 | 24297848 | [37] |
| 2. | Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance | Moreno-Navarrete, et al. | 2013 | 23436919 | [70] |
| 3. | Circulating irisin in relation to insulin resistance and the metabolic syndrome | Park, et al. | 2013 | 24057291 | [136] |
| 4. | Serum irisin levels and clinical implication in elderly patients with type 2 diabetes mellitus | Xuan, et al. | 2020 | 32849950 | [138] |
| 5. | Serum irisin levels, endothelial dysfunction, and inflammation in pediatric patients with type 2 diabetes mellitus and metabolic syndrome | Huerta-Delgado, et al. | 2020 | 32964051 | [139] |
| 6. | Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients | Crujeiras, et al. | 2014 | 24439241 | [142] |
| 7. | Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin | Palacios-González, et al. | 2015 | 25820255 | [143] |
| 8. | Effects of body weight reduction on serum irisin and metabolic parameters in obese subjects | Fukushima, et al. | 2016 | 27766246 | [144] |
| 9. | Effect of long-term moderate physical exercise on irisin between normal weight and obese men | Rashid, et al. | 2020 | 32952453 | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, N.; Zhao, Y.T.; Zhao, T.C. The Physiological Role of Irisin in the Regulation of Muscle Glucose Homeostasis. Endocrines 2021, 2, 266-283. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030025
Yano N, Zhao YT, Zhao TC. The Physiological Role of Irisin in the Regulation of Muscle Glucose Homeostasis. Endocrines. 2021; 2(3):266-283. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030025
Chicago/Turabian StyleYano, Naohiro, Yu Tina Zhao, and Ting C. Zhao. 2021. "The Physiological Role of Irisin in the Regulation of Muscle Glucose Homeostasis" Endocrines 2, no. 3: 266-283. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030025






