Clinical Aspects of Adolescent Endometriosis
Abstract
:1. Introduction
2. Prevalence and Social Context
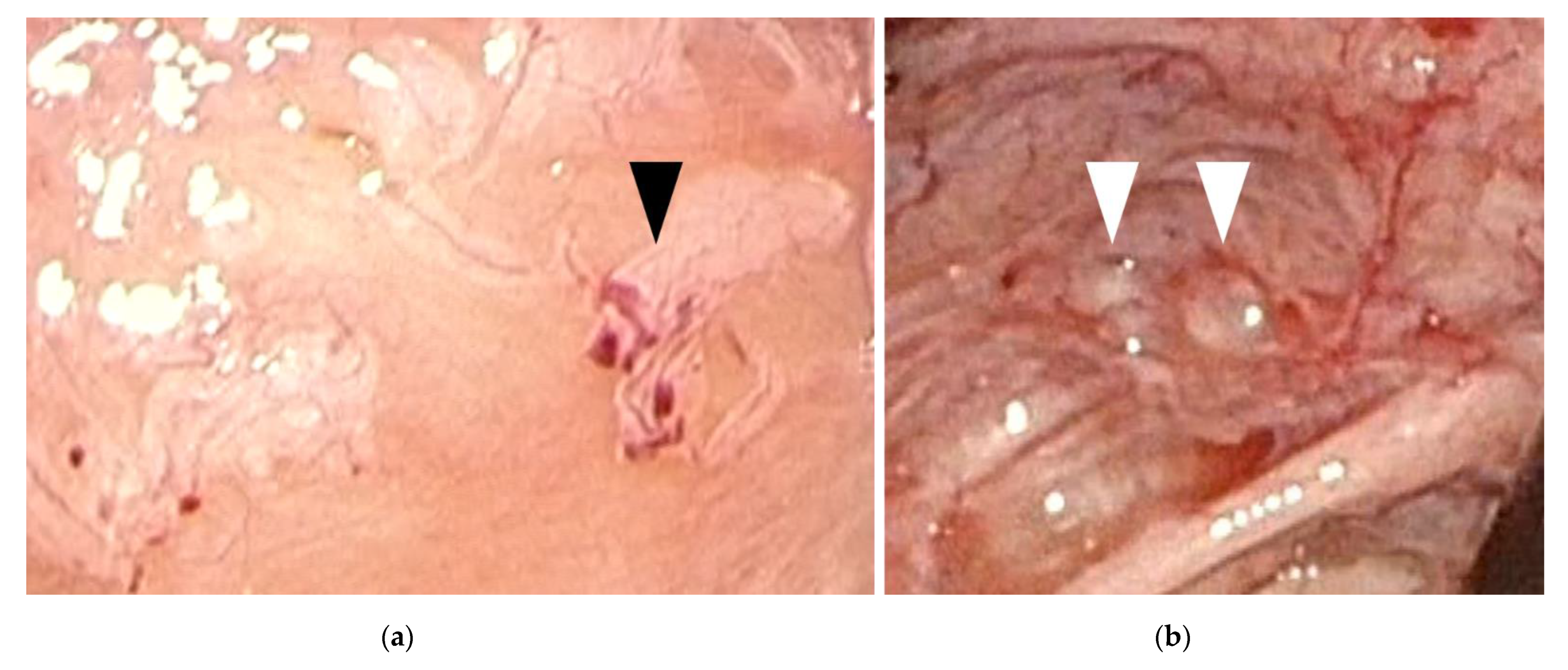
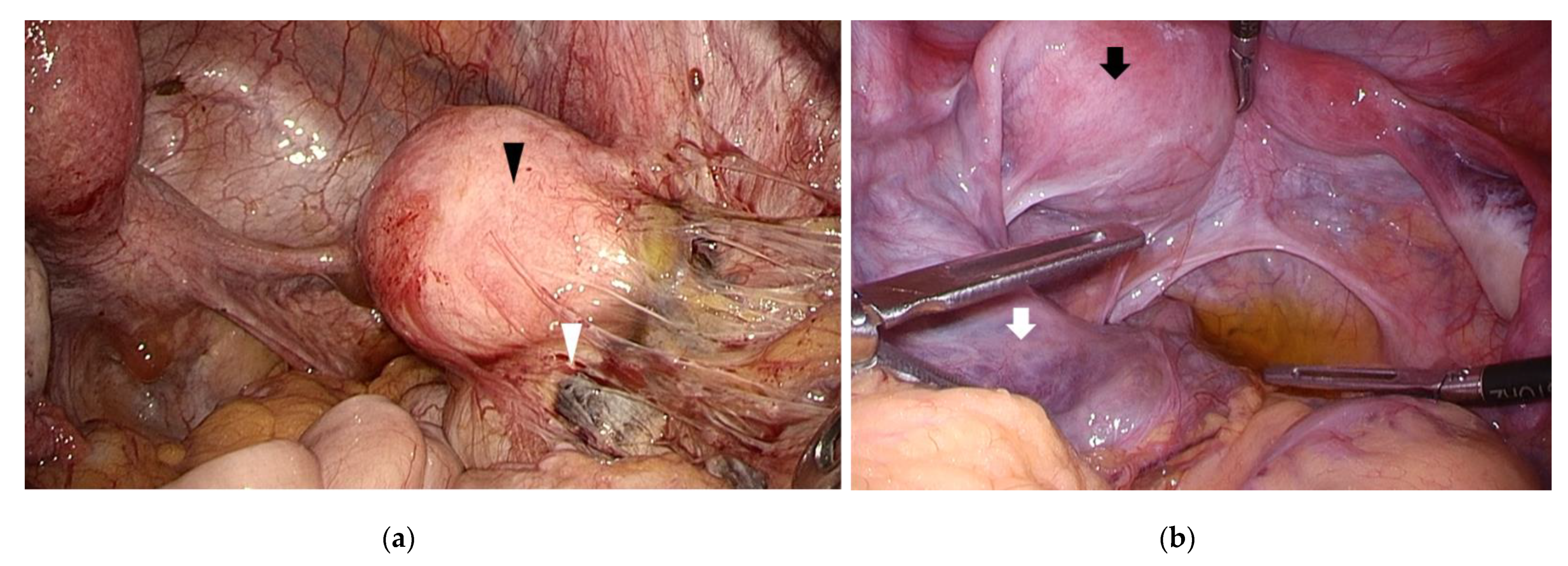
3. Clinical Findings
4. Pathology
4.1. Premenarcheal Endometriosis
4.2. Endometriosis Associated with Congenital Uterine Malformation and Outflow Obstruction
5. Treatment
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stuparich, M.A.; Donnellan, N.M.; Sanfilippo, J.S. Endometriosis in the Adolescent Patient. Semin. Reprod. Med. 2017, 35, 102–109. [Google Scholar] [PubMed]
- Gupta, J.; Cardoso, L.F.; Harris, C.S.; Dance, A.D.; Seckin, T.; Baker, N.; Ferguson, Y.O. How do adolescent girls and boys perceive symptoms suggestive of endometriosis among their peers? Findings from focus group discussions in New York City. BMJ Open 2018, 8, e020657. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.; Rijkers, A.; Hoppenbrouwers, K.; Meuleman, C.; D’Hooghe, T. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update 2013, 19, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martire, F.G.; Lazzeri, L.; Conway, F.; Siciliano, T.; Pietropolli, A.; Piccione, E.; Solima, E.; Centini, G.; Zupi, E.; Exacoustos, C. Adolescence and endometriosis: Symptoms, ultrasound signs and early diagnosis. Fertil. Steril. 2020, 114, 1049–1057. [Google Scholar] [CrossRef]
- Okaro, E.; Condous, G.; Khalid, A.; Timmerman, D.; Ameye, L.; Huffel, S.V.; Bourne, T. The use of ultrasound-based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain--can we reduce the need for laparoscopy? BJOG Int. J. Obstet. Gynaecol. 2006, 113, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gerges, B.; Lu, C.; Reid, S.; Chou, D.; Chang, T.; Condous, G. Sonographic evaluation of immobility of normal and endometriotic ovary in detection of deep endometriosis. Ultrasound Obstet. Gynecol. 2017, 49, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Redwine, D.B. Age-related evolution in color appearance of endometriosis. Fertil. Steril. 1987, 48, 1062–1063. [Google Scholar] [CrossRef]
- Laufer, M.R. Current approaches to optimizing the treatment of endometriosis in adolescents. Gynecol. Obstet. Investig. 2008, 66 (Suppl. S1), 19–27. [Google Scholar] [CrossRef]
- Benagiano, G.; Guo, S.-W.; Puttemans, P.; Gordts, S.; Brosens, I. Progress in the diagnosis and management of adolescent endometriosis: An opinion. Reprod. Biomed. Online 2018, 36, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, J.S.; Wakim, N.G.; Schikler, K.N.; Yussman, M.A. Endometriosis in association with uterine anomaly. Am. J. Obstet. Gynecol. 1986, 154, 39–43. [Google Scholar] [CrossRef]
- Saridogan, E. Adolescent endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 46–49. [Google Scholar] [CrossRef]
- Stavroulis, A.; Saridogan, E.; Creighton, S.; Cutner, A. Laparoscopic treatment of endometriosis in teenagers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 248–250. [Google Scholar] [CrossRef]
- Roman, J.D. Adolescent endometriosis in the Waikato region of New Zealand--a comparative cohort study with a mean follow-up time of 2.6 years. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 179–183. [Google Scholar] [CrossRef]
- Yeung, P., Jr.; Sinervo, K.; Winer, W.; Albee, R.B., Jr. Complete laparoscopic excision of endometriosis in teenagers: Is postoperative hormonal suppression necessary? Fertil. Steril. 2011, 95, 1909–1912.e1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, M.-L.; Seong, S.J.; Bae, J.W.; Cho, Y.J. Recurrence of Ovarian Endometrioma in Adolescents after Conservative, Laparoscopic Cyst Enucleation. J. Pediatr. Adolesc. Gynecol. 2017, 30, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Tandoi, I.; Somigliana, E.; Riparini, J.; Ronzoni, S.; Vigano, P.; Candiani, M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.P.; Fedele, L.; Arcaini, L.; Bianchi, S.; Rognoni, M.T.; Candiani, G.B. Laparoscopy in the diagnosis of chronic pelvic pain in adolescent women. J. Reprod. Med. 1989, 34, 827–830. [Google Scholar] [PubMed]
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.-M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Hirokawa, W.; Iwase, A.; Goto, M.; Takikawa, S.; Nagatomo, Y.; Nakahara, T.; Bayasula, B.; Nakamura, T.; Manabe, S.; Kikkawa, F. The post-operative decline in serum anti-Mullerian hormone correlates with the bilaterality and severity of endometriosis. Hum. Reprod. 2011, 26, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Kontoravdis, A.; Hassan, E.; Hassiakos, D.; Botsis, D.; Kontoravdis, N.; Creatsas, G. Laparoscopic evaluation and management of chronic pelvic pain during adolescence. Clin. Exp. Obstet. Gynecol. 1999, 26, 76–77. [Google Scholar]
- Laufer, M.; Goitein, L.; Bush, M.; Cramer, D.; Emans, S. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol. 1997, 10, 199–202. [Google Scholar] [CrossRef]
- Gałczyński, K.; Jóźwik, M.; Lewkowicz, D.; Semczuk-Sikora, A.; Semczuk, A. Ovarian endometrioma—A possible finding in adolescent girls and young women: A mini-review. J. Ovarian Res. 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matalliotakis, M.; Goulielmos, G.N.; Matalliotaki, C.; Trivli, A.; Matalliotakis, I.; Arici, A. Endometriosis in Adolescent and Young Girls: Report on a Series of 55 Cases. J. Pediatr. Adolesc. Gynecol. 2017, 30, 568–570. [Google Scholar] [CrossRef]
- Reid, S.; Lu, C.; Casikar, I.; Reid, G.; Abbott, J.; Cario, G.; Chou, D.; Kowalski, D.; Cooper, M.; Condous, G. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real-time dynamic transvaginal ultrasound technique: The sliding sign. Ultrasound Obstet. Gynecol. 2013, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal ‘tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audebert, A.; Lecointre, L.; Afors, K.; Koch, A.; Wattiez, A.; Akladios, C. Adolescent Endometriosis: Report of a Series of 55 Cases With a Focus on Clinical Presentation and Long-Term Issues. J. Minim. Invasive Gynecol. 2015, 22, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Redwine, D.B. The distribution of endometriosis in the pelvis by age groups and fertility. Fertil. Steril. 1987, 47, 173–175. [Google Scholar] [CrossRef]
- Davis, G.D.; Thillet, E.; Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 1993, 14, 362–368. [Google Scholar] [CrossRef]
- Jansen, R.P.; Russell, P. Nonpigmented endometriosis: Clinical, laparoscopic, and pathologic definition. Am. J. Obstet. Gynecol. 1986, 155, 1154–1159. [Google Scholar] [CrossRef]
- Laufer, M.R. Identification of clear vesicular lesions of atypical endometriosis: A new technique. Fertil. Steril. 1997, 68, 739–740. [Google Scholar] [CrossRef]
- Marsh, E.E.; Laufer, M.R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 2005, 83, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Fuhr, N.; David, M.; Schneppel, L.; Papadopoulos, T. Histological confirmation of endometriosis in a 9-year-old girl suffering from unexplained cyclic pelvic pain since her eighth year of life. Gynecol. Obstet. Invest. 2009, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Gogacz, M.; Sarzyński, M.; Napierała, R.; Sierocińska-Sawa, J.; Semczuk, A. Ovarian endometrioma in an 11-year-old girl before menarche: A case study with literature review. J. Pediatr. Adolesc. Gynecol. 2012, 25, e5–e7. [Google Scholar] [CrossRef]
- Lin, J.; Xiang, D.; Zhang, J.-L.; Allickson, J.; Xiang, C. Plasticity of human menstrual blood stem cells derived from the endometrium. J. Zhejiang Univ. Sci. B 2011, 12, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Figueira, P.G.M.; Abrão, M.S.; Krikun, G.; Taylor, H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosens, I.; Puttemans, P.; Benagiano, G. Endometriosis: A life cycle approach? Am. J. Obstet. Gynecol. 2013, 209, 307–316. [Google Scholar] [CrossRef]
- Huber, A. The frequency of physiologic vaginal bleeding of newborn infants. Zentralbl. Gynakol. 1976, 98, 1017–1020. [Google Scholar]
- Fluhmann, C.F. The developmental anatomy of the cervix uteri. Obstet. Gynecol. 1960, 15, 62–69. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar] [PubMed]
- Song, X.-C.; Yu, X.; Luo, M.; Yu, Q.; Zhu, L. Clinical Characteristics and Postoperative Symptoms of 85 Adolescents with Endometriosis. J. Pediatr. Adolesc. Gynecol. 2020, 33, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Olive, D.L.; Henderson, D.Y. Endometriosis and mullerian anomalies. Obstet. Gynecol. 1987, 69, 412–415. [Google Scholar]
- Yang, Y.; Wang, Y.; Yang, J.; Wang, S.; Lang, J. Adolescent endometriosis in China: A retrospective analysis of 63 cases. J. Pediatr. Adolesc. Gynecol. 2012, 25, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gordts, S.; Benagiano, G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum. Reprod. 2013, 28, 2026–2031. [Google Scholar] [CrossRef] [Green Version]
- Nanda, K.; Lendvay, A.; Kwok, C.; Tolley, E.; Dubé, K.; Brache, V. Continuous compared with cyclic use of oral contraceptive pills in the Dominican Republic: A randomized controlled trial. Obstet. Gynecol. 2014, 123, 1012–1022. [Google Scholar] [CrossRef]
- Steenberg, C.K.; Tanbo, T.G.; Qvigstad, E. Endometriosis in adolescence: Predictive markers and management. Acta Obstet. Gynecol. Scand. 2013, 92, 491–495. [Google Scholar] [CrossRef]
- DiVasta, A.D.; Laufer, M.R. The use of gonadotropin releasing hormone analogues in adolescent and young patients with endometriosis. Curr. Opin. Obstet. Gynecol. 2013, 25, 287–292. [Google Scholar] [CrossRef]
- Laufer, M.R. Helping “adult gynecologists” diagnose and treat adolescent endometriosis: Reflections on my 20 years of personal experience. J. Pediatr. Adolesc. Gynecol. 2011, 24, S13–S17. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Schwartz, B.I.; Alexander, M.; Breech, L.L. Levonorgestrel Intrauterine Device Use for Medical Indications in Nulliparous Adolescents and Young Adults. J. Adolesc. Health 2021, 68, 357–363. [Google Scholar] [CrossRef]
- ACOG Practice bulletin, no. 114: Management of endometriosis. Obstet. Gynecol. 2010, 116, 223–236. [Google Scholar]
- Fu, J.; Song, H.; Zhou, M.; Zhu, H.; Wang, Y.; Chen, H.; Huang, W. Progesterone receptor modulators for endometriosis. Cochrane Database Syst. Rev. 2017, 25, CD009881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X. Effect of mifepristone in the different treatments of endometriosis. Clin. Exp. Obstet. Gynecol. 2016, 43, 350–353. [Google Scholar]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind the therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of Anti-VEGF/VEGFR agents on animal models of endometriosis: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, K.; Llarena, N.C.; Rehmer, J.M.; Richards, E.; Falcone, T. The role of pharmacotherapy in the treatment of endometriosis across the lifespan. Expert Opin. Pharmacother. 2020, 21, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Dowlut-McElroy, T.; Strickland, J.L. Endometriosis in adolescents. Curr. Opin. Obstet. Gynecol. 2017, 29, 306–309. [Google Scholar] [CrossRef]
- Vercellini, P.; Crosignani, P.; Somigliana, E.; Viganò, P.; Frattaruolo, M.P.; Fedele, L. “Waiting for Godot”: A commonsense approach to the medical treatment of endometriosis. Hum. Reprod. 2011, 26, 3–13. [Google Scholar] [CrossRef] [Green Version]
| Symptoms—Atypical | ||
| More often acyclic pain [3] | ||
| Frequently resistant to combined oral contraceptive (COC) and NSAIDs [3] | ||
| Findings—Lesions less evident | ||
| Ultrasound | ||
| Small endometriomas—persistence beyond at least 3 menstrual cycles may assist in judgement [4] | ||
| Earlier-stage superficial tissue invasion—site-specific tenderness and reduced ovarian mobility may aid in diagnosis [5,6] | ||
| Laparoscopy | ||
| Red/clear peritoneal lesions and vesicular lesions—filling pelvis with saline and submersing laparoscope may assist in diagnosis [7,8] | ||
| Pathology—distinct classifications | ||
| Premenarcheal endometriosis—neonatal uterine bleeding (NUB) considered as potential source [9] | ||
| Endometriosis associated with congenital uterine malformation and outflow obstruction—surgical correction is necessary [10] | ||
| Treatment | ||
| Medication | ||
| COC/NSAIDs—mainstream of treatment [11] | ||
| GnRH agonists/antagonists—should be postponed until 17 years of age [11] | ||
| Surgery | ||
| Mostly good prognosis reported on pain [12,13,14] | ||
| High recurrence rates [15,16] | ||
| No consensus regarding best timing for surgical intervention [17,18,19,20] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T. Clinical Aspects of Adolescent Endometriosis. Endocrines 2021, 2, 301-310. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030028
Nakamura T. Clinical Aspects of Adolescent Endometriosis. Endocrines. 2021; 2(3):301-310. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030028
Chicago/Turabian StyleNakamura, Tomoko. 2021. "Clinical Aspects of Adolescent Endometriosis" Endocrines 2, no. 3: 301-310. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030028





