Paradoxical Relationships between Serum 25(OH)D and Ferritin with Body Composition and Burnout: Variation by Sex and Sports Team
Abstract
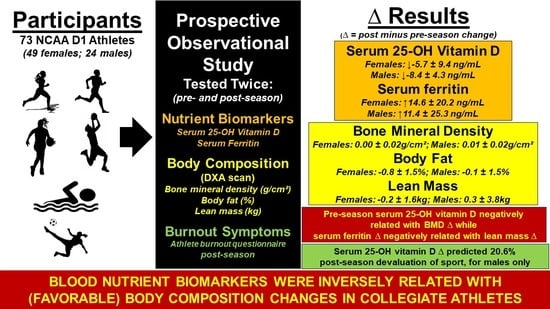
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Pasricha, S.R.; Low, M.; Thompson, J.; Farrell, A.; De-Regil, L.M. Iron supplementation benefits physical performance in women of reproductive age: A systematic review and meta-analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Lansdowne, A.T.; Provost, S.C. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology 1998, 135, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, A.; Jenoure, P.J.; Engebretsen, L.; Alonso, J.M.; Bahr, R.; Clough, A.F.; de Bondt, G.; Dvorak, J.; Maloley, R.; Matheson, G.; et al. The International Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes, March 2009. Clin. J. Sport Med. 2009, 19, 347–365. [Google Scholar] [CrossRef] [Green Version]
- Rockwell, M.; Hulver, M.; Eugene, E. Vitamin D Practice Patterns in National Collegiate Athletic Association Division I Collegiate Athletics Programs. J. Athl. Train. 2020, 55, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron Deficiency and Anemia among Collegiate Athletes: A Retrospective Chart Review. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef]
- Hackney, A.C.; Elliott-Sale, K.J. Exercise Endocrinology: “What Comes Next?”. Endocrines 2021, 2, 17. [Google Scholar] [CrossRef]
- Grieshober, J.A.; Mehran, N.; Photopolous, C.; Fishman, M.; Lombardo, S.J.; Kharrazi, F.D. Vitamin D Insufficiency Among Professional Basketball Players: A Relationship to Fracture Risk and Athletic Performance. Orthop. J. Sports Med. 2018, 6, 2325967118774329. [Google Scholar] [CrossRef] [Green Version]
- Villacis, D.; Yi, A.; Jahn, R.; Kephart, C.J.; Charlton, T.; Gamradt, S.C.; Romano, R.; Tibone, J.E.; Hatch, G.F., 3rd. Prevalence of Abnormal Vitamin D Levels Among Division I NCAA Athletes. Sports Health 2014, 6, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.B.; Payne, D.C.; Gallo, S.; Busteed, D.R.; Jones, M.T. Vitamin D Status Differs by Sex, Sport-Season, and Skin Pigmentation among Elite Collegiate Basketball Players. Sports 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, J.B.; Gallo, S.; Worswick, J.M.; Busteed, D.R.; Jones, M.T. 25-Hydroxyvitamin D, Vitamin D Binding Protein, Bioavailable 25-Hydroxyvitamin D, and Body Composition in a Diverse Sample of Women Collegiate Indoor Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.E.; Bolia, I.K.; Korber, S.; Mayfield, C.K.; Lindsay, A.; Rosen, J.; McMannes, S.; Romano, R.; Tibone, J.E.; Gamradt, S.C. Five-Year Surveillance of Vitamin D Levels in NCAA Division I Football Players: Risk Factors for Failed Supplementation. Orthop J. Sports Med. 2021, 9, 2325967120975100. [Google Scholar] [CrossRef] [PubMed]
- Halliday, T.M.; Peterson, N.J.; Thomas, J.J.; Kleppinger, K.; Hollis, B.W.; Larson-Meyer, D.E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med. Sci. Sports Exerc. 2011, 43, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Cowell, B.S.; Rosenbloom, C.A.; Skinner, R.; Summers, S.H. Policies on screening female athletes for iron deficiency in NCAA division I-A institutions. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 277–285. [Google Scholar] [CrossRef]
- Cook, J.D.; Skikne, B.S. Iron deficiency: Definition and diagnosis. J. Intern. Med. 1989, 226, 349–355. [Google Scholar] [CrossRef]
- Eichner, E.R. Perennial questions: On fatigue, on iron, and on anemia. Curr Sports Med. Rep. 2012, 11, 274–275. [Google Scholar] [CrossRef]
- Pilch, W.; Kita, B.; Piotrowska, A.; Tota, Ł.; Maciejczyk, M.; Czerwińska-Ledwig, O.; Sadowska-Krepa, E.; Kita, S.; Pałka, T. The effect of vitamin D supplementation on the muscle damage after eccentric exercise in young men: A randomized, control trial. J. Int. Soc. Sports Nutr. 2020, 17, 53. [Google Scholar] [CrossRef]
- Miller, J.R.; Dunn, K.W.; Ciliberti, L.J., Jr.; Patel, R.D.; Swanson, B.A. Association of Vitamin D With Stress Fractures: A Retrospective Cohort Study. J. Foot Ankle Surg. 2016, 55, 117–120. [Google Scholar] [CrossRef]
- Risser, W.L.; Lee, E.J.; Poindexter, H.B.; West, M.S.; Pivarnik, J.M.; Risser, J.M.; Hickson, J.F. Iron deficiency in female athletes: Its prevalence and impact on performance. Med. Sci. Sports Exerc. 1988, 20, 116–121. [Google Scholar] [CrossRef]
- Barakat, C.; Pearson, J.; Escalante, G.; Campbell, B.; De Souza, E.O. Body Recomposition: Can Trained Individuals Build Muscle and Lose Fat at the Same Time? Strength Cond. J. 2020, 42, 7–21. [Google Scholar] [CrossRef]
- Bennell, K.L.; Malcolm, S.A.; Khan, K.M.; Thomas, S.A.; Reid, S.J.; Brukner, P.D.; Ebeling, P.R.; Wark, J.D. Bone mass and bone turnover in power athletes, endurance athletes, and controls: A 12-month longitudinal study. Bone 1997, 20, 477–484. [Google Scholar] [CrossRef]
- Stanfa, M.R.; Silles, N.N.; Cooper, A.; Arena, S.; Landis-Piwowar, K.; Aprik, C.; Hew-Butler, T. Risk Factors for Collegiate Swimmers Hospitalized With Exertional Rhabdomyolysis. Clin. J. Sport Med. 2017, 27, 37–45. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Carlson, J.L.; Chang, A.; Sainani, K.L.; Shultz, R.; Kim, J.H.; Cutti, P.; Golden, N.H.; Fredericson, M. Association of the Female Athlete Triad Risk Assessment Stratification to the Development of Bone Stress Injuries in Collegiate Athletes. Am. J. Sports Med. 2017, 45, 302–310. [Google Scholar] [CrossRef]
- Scheid, J.L.; Stefanik, M.E. Drive for Thinness Predicts Musculoskeletal Injuries in Division II NCAA Female Athletes. J. Funct. Morphol. Kinesiol. 2019, 4, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, M.A.; Houghton, L.A.; Reeder, A.I.; Harper, M.J.; Conner, T.S. Serum 25-hydroxyvitamin D concentrations and depressive symptoms among young adult men and women. Nutrients 2014, 6, 4720–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahdat Shariatpanaahi, M.; Vahdat Shariatpanaahi, Z.; Moshtaaghi, M.; Shahbaazi, S.H.; Abadi, A. The relationship between depression and serum ferritin level. Eur. J. Clin. Nutr. 2007, 61, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.; Nachtigall, D. Iron supplementation in athletes. Current recommendations. Sports Med. 1998, 26, 207–216. [Google Scholar] [CrossRef]
- Hologic. QDR Reference Manual, 2nd ed.; MAN-00732; Hologic, Inc.: Bedford, MA, USA, 2007. [Google Scholar]
- Raedeke, T.D.; Smith, A.L. Development and Preliminary Validation of an Athlete Burnout Measure. J. Sport Exerc. Psychol. 2001, 23, 281–306. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Lerchbaum, E.; Pilz, S.; Trummer, C.; Schwetz, V.; Pachernegg, O.; Heijboer, A.C.; Obermayer-Pietsch, B. Vitamin D and Testosterone in Healthy Men: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 4292–4302. [Google Scholar] [CrossRef]
- Krzywański, J.; Pokrywka, A.; Młyńczak, M.; Mikulski, T. Is vitamin D status reflected by testosterone concentration in elite athletes? Biol. Sport 2020, 37, 229–237. [Google Scholar] [CrossRef]
- Fraser, I.S.; Warner, P.; Marantos, P.A. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet. Gynecol. 2001, 98, 806–814. [Google Scholar] [CrossRef]
- Wilson-Barnes, S.L.; Hunt, J.E.A.; Williams, E.L.; Allison, S.J.; Wild, J.J.; Wainwright, J.; Lanham-New, S.A.; Manders, R.J.F. Seasonal variation in vitamin D status, bone health and athletic performance in competitive university student athletes: A longitudinal study. J. Nutr. Sci. 2020, 9, e8. [Google Scholar] [CrossRef] [Green Version]
- Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin. J. Sport Med. 2010, 20, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.M.; Redzic, M.; Thomas, D.T. The effects of season-long vitamin D supplementation on collegiate swimmers and divers. Int J. Sport Nutr. Exerc. Metab. 2013, 23, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klesges, R.C.; Ward, K.D.; Shelton, M.L.; Applegate, W.B.; Cantler, E.D.; Palmieri, G.M.; Harmon, K.; Davis, J. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA 1996, 276, 226–230. [Google Scholar] [CrossRef]
- Heller, J.E.; Thomas, J.J.; Hollis, B.W.; Larson-Meyer, D.E. Relation between vitamin D status and body composition in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 128–135. [Google Scholar] [CrossRef]
- LeFevre, M.L.; LeFevre, N.M. Vitamin D Screening and Supplementation in Community-Dwelling Adults: Common Questions and Answers. Am. Fam. Physician 2018, 97, 254–260. [Google Scholar]
- Jin, J. Screening for Vitamin D Deficiency in Adults. JAMA 2021, 325, 1480. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comassi, M.; Vitolo, E.; Pratali, L.; Del Turco, S.; Dellanoce, C.; Rossi, C.; Santini, E.; Solini, A. Acute effects of different degrees of ultra-endurance exercise on systemic inflammatory responses. Intern. Med. J. 2015, 45, 74–79. [Google Scholar] [CrossRef]
- Deruisseau, K.C.; Roberts, L.M.; Kushnick, M.R.; Evans, A.M.; Austin, K.; Haymes, E.M. Iron status of young males and females performing weight-training exercise. Med. Sci. Sports Exerc. 2004, 36, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zürcher, S.J.; Quadri, A.; Huber, A.; Thomas, L.; Close, G.L.; Brunner, S.; Noack, P.; Gojanovic, B.; Kriemler, S. Predictive Factors for Vitamin D Concentrations in Swiss Athletes: A Cross-sectional Study. Sports Med. Int. Open 2018, 2, E148–E156. [Google Scholar] [CrossRef] [Green Version]




| Team (N = 73) | Age (years) | Height (m) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| ♀XC (n = 16) | 19.4 ± 1.5 | 1.64 ± 0.1 | 56.6 ± 4.9 | 20.8 ± 1.8 |
| ♂XC (n = 9) | 20.3 ± 0.9 | 1.80 ± 0.1 | 68.8 ± 6.9 | 21.3 ± 0.9 |
| ♀Swim (n-12) | 19.6 ± 1.1 | 1.70 ± 0.1 | 67.2 ± 5.8 | 23.2 ± 2.1 |
| ♂Swim (n = 6) | 20.7 ± 1.2 | 1.84 ± 0.1 | 78.5 ± 5.2 | 23.3 ± 1.6 |
| ♀Basketball (n = 10) | 19.7 ± 1.4 | 1.76 ± 0.1 | 75.1 ± 12.0 | 24.3 ± 3.4 |
| ♂Basketball (n = 9) | 20.1 ± 1.5 | 1.96 ± 0.1 | 94.5 ± 14.3 | 24.6 ± 2.8 |
| ♀Soccer (n = 11) | 19.5 ± 1.2 | 1.69 ± 0.1 | 64.9 ± 14.4 | 22.6 ± 3.6 |
| ♀COMBINED (n = 49) | 19.5 ± 1.3 | 1.69 ± 0.1 | 64.8 ± 11.5 | 22.6 ± 2.0 |
| ♂COMBINED (n = 24) | 20.3 ± 0.1 | 1.87 ± 0.1 | 80.9 ± 15.0 | 23.0 ± 2.4 |
| Team (N = 73) | Pre Body Fat (%) | ∆ Body Fat (%) | Pre BMD (g/cm2) | ∆ BMD (g/cm2) | Pre Lean Mass (kg) | ∆ Lean Mass (kg) | T or Z-Score |
|---|---|---|---|---|---|---|---|
| ♀XC (n = 16) | 18.7 ± 3.1 | −0.8 ± 1.1 | 1.08 ± 0.06 | −0.00 ± 0.02 | 43.7 ± 4.3 | −0.4 ± 0.9 | −0.34 ± 0.82 |
| ♂XC (n = 9) | 8.5 ± 1.1 | −0.1 ± 0.8 | 1.22 ± 0.05 | −0.01 ± 0.01 | 59.4 ± 6.1 | −0.3 ± 0.9 | −0.19 ± 0.46 |
| ♀Swim (n-12) | 21.2 ± 3.8 | −1.7 ± 1.8 | 1.08 ± 0.07 | 0.00 ± 0.02 | 49.0 ± 3.0 | 1.8 ± 1.3 | −0.22 ± 0.76 |
| ♂Swim (n = 6) | 12.0 ± 2.4 | −1.3 ± 1.7 | 1.17 ± 0.07 | 0.00 ± 0.01 | 66.0 ± 5.5 | 0.5 ± 2.1 | −0.57 ± 0.75 |
| ♀Basketball (n = 10) | 18.2 ± 5.1 | −0.7 ± 1.1 | 1.19 ± 0.09 | 0.01 ± 0.02 | 56.1 ± 5.9 | −0.6 ± 1.9 | 1.10 ± 0.55 |
| ♂Basketball (n = 9) | 10.7 ± 3.0 | 0.8 ± 1.4 | 1.37 ± 0.09 | 0.02 ± 0.02 | 77.8 ± 9.1 | −1.3 ± 1.4 | 1.14 ± 0.79 |
| ♀Soccer (n = 11) | 19.9 ± 5.0 | 0.2 ± 1.5 | 1.09 ± 0.07 | 0.01 ± 0.01 | 48.7 ± 6.5 | −0.8 ± 1.5 | 0.76 ± 0.43 |
| ♀COMBINED (n = 49) | 19.5 ± 4.2 | −0.8 ± 1.5 | 1.10 ± 0.08 | 0.00 ± 0.02 | 48.7 ± 6.6 | −0.2 ± 1.6 | 0.18 ± 0.90 |
| ♂COMBINED (n = 24) | 10.2 ± 2.6 | −0.1 ± 1.5 | 1.27 ± 0.11 | 0.01 ± 0.02 | 67.9 ± 10.8 | 0.3 ± 3.8 | 0.17 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hew-Butler, T.; Aprik, C.; Byrd, B.; Landis-Piwowar, K.; Smith-Hale, V.; VanSumeren, M.; Sabourin, J.; Byrd, G.; Martin, J. Paradoxical Relationships between Serum 25(OH)D and Ferritin with Body Composition and Burnout: Variation by Sex and Sports Team. Endocrines 2021, 2, 320-333. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030030
Hew-Butler T, Aprik C, Byrd B, Landis-Piwowar K, Smith-Hale V, VanSumeren M, Sabourin J, Byrd G, Martin J. Paradoxical Relationships between Serum 25(OH)D and Ferritin with Body Composition and Burnout: Variation by Sex and Sports Team. Endocrines. 2021; 2(3):320-333. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030030
Chicago/Turabian StyleHew-Butler, Tamara, Carrie Aprik, Brigid Byrd, Kristin Landis-Piwowar, Valerie Smith-Hale, Matthew VanSumeren, Jordan Sabourin, Gregory Byrd, and Jeffrey Martin. 2021. "Paradoxical Relationships between Serum 25(OH)D and Ferritin with Body Composition and Burnout: Variation by Sex and Sports Team" Endocrines 2, no. 3: 320-333. https://0-doi-org.brum.beds.ac.uk/10.3390/endocrines2030030