Pd-Based Polysaccharide Hydrogels as Heterogeneous Catalysts for Oxidation of Aromatic Alcohols
Abstract
:1. Introduction
2. Experimental Section
2.1. Polysaccharides Catalyst and Reagents
2.2. Preparation of Hydrogel Beads with Calcium Chloride (I-CaCl2)
2.3. Preparation of Hydrogel Beads with Chitosan (I-C)
2.4. Reaction Procedure
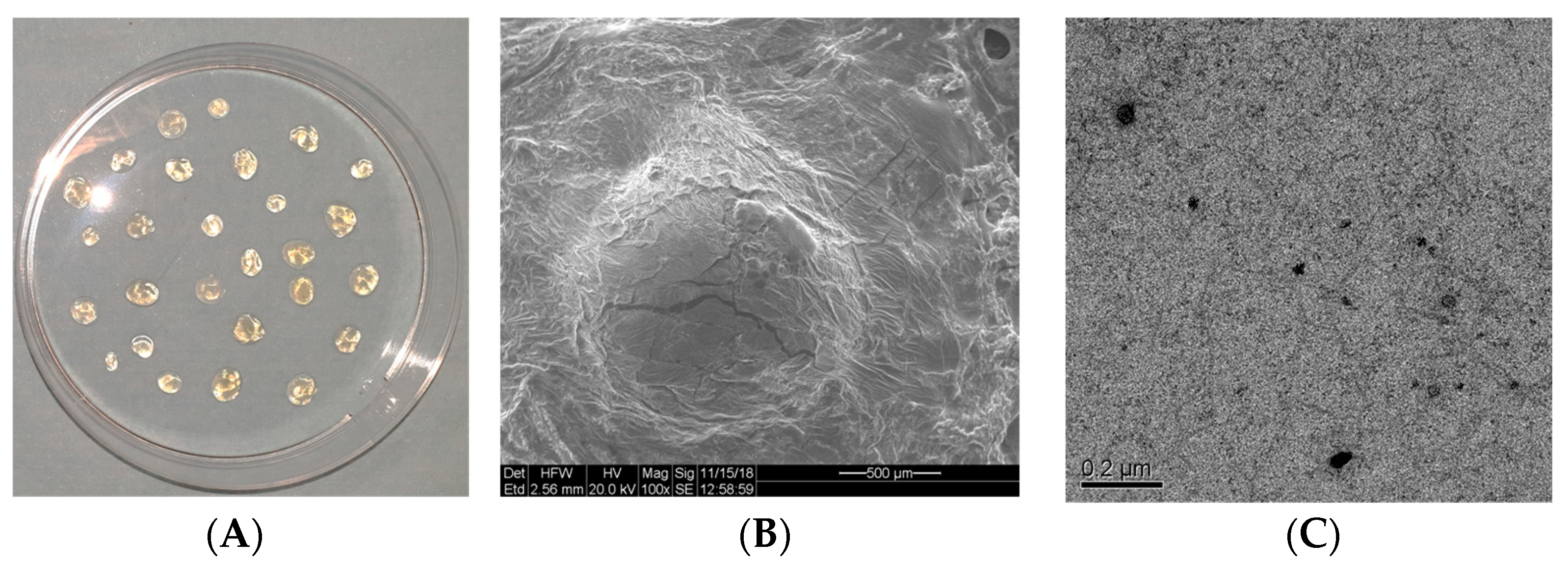
2.5. Scanning Electron Microscope (SEM) Analysis
2.6. High-Resolution Transmission Electron Microscopic (HRTEM) Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Moleculs 2020, 25, 3156. [Google Scholar] [CrossRef]
- Quignard, F.; Di Renzo, F.; Guibal, E. From natural polysaccharides to materials for catalysis, adsorption, and remediation. In Carbohydrates in Sustainable Development I; Springer: Berlin/Heidelberg, Germany, 2010; pp. 165–197. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of ordered structure in polysaccharide hydrogel: A review. Carbohydr. Polym. 2019, 205, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Naim, S.; Samuel, B.; Chauhan, B.; Paradkar, A. Effect of potassium chloride and cationic drug on swelling, erosion and release from κ-carrageenan matrices. AAPS PharmSciTech 2004, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.K.; Sahoo, D.; Nayak, P.L. Chitosan-sodium alginate nanocomposites blended with cloisite 30B as a novel drug delivery system for anticancer drug curcumin. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 402–411. [Google Scholar]
- Leone, G.; Barbucci, R. Polysaccharide based hydrogels for biomedical applications. In Hydrogels; Springer: Milan, Italy, 2009; pp. 25–41. [Google Scholar]
- Magnani, A.; Rappuoli, R.; Lamponi, S.; Barbucci, R. Novel polysaccharide hydrogels: Characterization and properties. Polym. Adv. Technol. 2000, 11, 488–495. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Blakemore, W.R.; Harpell, A.R. Carrageenan. In Food Stabilisers, Thickeners and Gelling Agents; John Wiley & Sons: New York, NY, USA, 2010; pp. 73–94. [Google Scholar]
- Reddy, K.R.; Rajgopal, K.; Maheswari, C.U.; Kantam, M.L. Chitosan hydrogel: A green and recyclable biopolymer catalyst for aldol and Knoevenagel reactions. N. J. Chem. 2006, 30, 1549–1552. [Google Scholar] [CrossRef]
- Wolfson, A.; Levy-Ontman, O. Recent Developments in the Immobilization of Palladium Complexes on Renewable Polysaccharides for Suzuki–Miyaura Cross-Coupling of Halobenzenes and Phenylboronic Acids. Catalysts 2020, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, A.; Levy-Ontman, O. Development and application of palladium nanoparticles on renewable polysaccharides as catalysts for the Suzuki cross-coupling of halobenzenes and phenylboronic acids. Mol. Catal. 2020, 493, 111048. [Google Scholar] [CrossRef]
- Kadokawa, J.-I. Enzymatic preparation of functional polysaccharide hydrogels by phosphorylase catalysis. Pure Appl. Chem. 2018, 90, 1045–1054. [Google Scholar] [CrossRef]
- Bogdanova, L.R.; Rogov, A.M.; Zueva, O.S.; Zuev, Y.F. Lipase enzymatic microreactor in polysaccharide hydrogel: Structure and properties. Russ. Chem. Bull. 2019, 68, 400–404. [Google Scholar] [CrossRef]
- Nurettin, S. Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci. 2013, 38, 1329–1356. [Google Scholar]
- Levy-Ontman, O.; Biton, S.; Shlomov, B.; Wolfson, A. Renewable polysaccharides as supports for palladium phosphine catalysts. Polymers 2018, 10, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfson, A.; Biton, S.; Levy-Ontman, O. Study of Pd-based catalysts within red algae-derived polysaccharide supports in a Suzuki cross-coupling reaction. RSC Adv. 2018, 8, 37939–37948. [Google Scholar] [CrossRef] [Green Version]
- Leviev, S.; Wolfson, A.; Levy-Ontman, O. Novel iota carrageenan-based RhCl3 as an efficient and recyclable catalyst in Suzuki cross coupling. Mol. Catal. 2020, 486, 110841. [Google Scholar] [CrossRef]
- Leviev, S.; Wolfson, A.; Levy-Ontman, O. RhCl(TPPTS)3 supported on iota-carrageenan as recyclable catalysts for Suzuki cross-coupling. J. Appl. Polym. Sci. 2019, 136, 48200–48204. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Blum, D.; Golden, R.; Pierschel, E.; Leviev, S.; Wolfson, A. Palladium Based-Polysaccharide Hydrogels as Catalysts in the Suzuki Cross-Coupling Reaction. J. Inorg. Organomet. Polym. Mater. 2019, 30, 622–636. [Google Scholar] [CrossRef]
- Stamker, E.; Levy-Ontman, O.; Wolfson, A. Green procedure for aerobic oxidation of benzylic alcohols with palladium supported on iota-carrageenan in ethanol. Polymers 2021, 13, 498. [Google Scholar] [CrossRef]
- Bäckvall, J.E. Modern Oxidation Methods; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.; Alegria, E.C.; Martins, N.M.; Martins, L.M.; Pombeiro, A.J. Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 91–174. [Google Scholar]
- Iwabuchi, Y. Green Oxidative Synthesis of Aldehydes and Ketones. In Green Oxidation in Organic Synthesis; Ning, J., Stahl, S.S., Eds.; John Wiley & Sons: New York, NY, USA, 2019; pp. 35–78. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Ueno, M.; Ohmura, S.D.; Wada, M.; Miyoshi, N. Aerobic oxidation of alcohols using bismuth bromide as a catalyst. Tetrahedron Lett. 2017, 60, 570–573. [Google Scholar] [CrossRef]
- Landaeta, V.R.; Rodríguez-Lugo, R.E. Aerobic oxidation reactions in the fine chemicals and pharmaceutical industries. In Catalytic Aerobic Oxidations; Royal Society of Chemistry: Cambridge, UK, 2020; pp. 252–290. [Google Scholar]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]

| Entry | Solvent | RP b | Pd(OAc)2 | Pd(OAc)2(TPPTS)2 | I-CaCl2 | I-C |
|---|---|---|---|---|---|---|
| 1 | Ethanol | 0.654 | 50 | 40 (78) c | 25 | 4 |
| 2 | Ethyl acetate | 0.228 | 26 | 28 (59) c | 16 | 3 |
| 3 | Petroleum ether | 0.111 | 31 | 25 (55) c | 38 | 14 |
| 4 | Hexane | 0.009 | 48 | 47 (86) c | 66 | 35 |
| Polysaccharide | Branched/Linear | Building Block | Functional Groups | Conversion (%) |
|---|---|---|---|---|
| Iota (I) | Linear | d-Gal-4-sulfate,3,6-anhydro-d-Gal-2-sulfate | –OH, –OSO3− | 66 71 b 40 c |
| Kappa (K) | Linear | d-Gal-4-sulfate,3,6-anhydro-d-Gal | –OH, –OSO3− | 44 |
| Alginate (A) | Linear | β-(1 → 4)-linked mannose and α-L guluronate | –OH, –COO− | 34 |
| Guar gum (G) | Branched | β-(1 → 4)-linked mannose | –OH | - |
| Entry | Mixing | Substrate | Pressure (atm) | Cycle | Conversion (%) |
|---|---|---|---|---|---|
| 1 | Stirring | Benzyl alcohol | 1 | 1 | 66 |
| 2 | Stirring | Benzyl alcohol | 1 | 2 | 33 |
| 3 | Stirring | Benzyl alcohol | 1 | 3 | 10 |
| 4 | Shaking | Benzyl alcohol | 1 | 1 | 49 |
| 5 | Shaking | Benzyl alcohol | 1 | 2 | 28 |
| 6 | Shaking | Benzyl alcohol | 1 | 3 | 25 |
| 7 | Stirring | Benzyl alcohol | 1 | 1 | 22 |
| 8 | Stirring | Benzyl alcohol | 3.8 | 1 | 69 |
| 9 | Stirring | Benzyl alcohol | 1 b | 1 | 60 |
| 10 | Stirring | Benzyl alcohol | 3.8 b | 1 | 55 |
| 11 | Stirring | 1-Phenyl ethanol | 1 | 1 | 63 |
| 12 | Stirring | 4-Methylbenzyl alcohol | 1 | 1 | 69 |
| 13 | Stirring | 4-Methoxylbenzyl alcohol | 1 | 1 | 99 |
| 14 | Stirring | Benzyl alcohol c | 1 | 1 | 25 |
| 15 | Stirring | Benzyl alcohol c | 1 | 2 | 23 |
| 16 | Stirring | Benzyl alcohol c | 1 | 3 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy-Ontman, O.; Stamker, E.; Mor, V.; Wolfson, A. Pd-Based Polysaccharide Hydrogels as Heterogeneous Catalysts for Oxidation of Aromatic Alcohols. Organics 2021, 2, 50-56. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010005
Levy-Ontman O, Stamker E, Mor V, Wolfson A. Pd-Based Polysaccharide Hydrogels as Heterogeneous Catalysts for Oxidation of Aromatic Alcohols. Organics. 2021; 2(1):50-56. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010005
Chicago/Turabian StyleLevy-Ontman, Oshrat, Eliraz Stamker, Vital Mor, and Adi Wolfson. 2021. "Pd-Based Polysaccharide Hydrogels as Heterogeneous Catalysts for Oxidation of Aromatic Alcohols" Organics 2, no. 1: 50-56. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010005






