The Role of the Catalyst on the Reactivity and Mechanism in the Diels–Alder Cycloaddition Step of the Povarov Reaction for the Synthesis of a Biological Active Quinoline Derivative: Experimental and Theoretical Investigations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Study
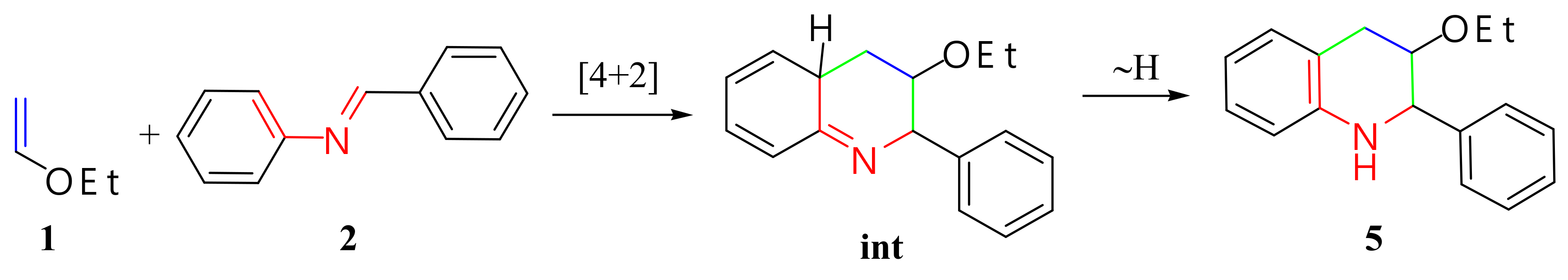
2.1.1. Imine
2.1.2. Quinoline Derivative
2.2. Biological Activity
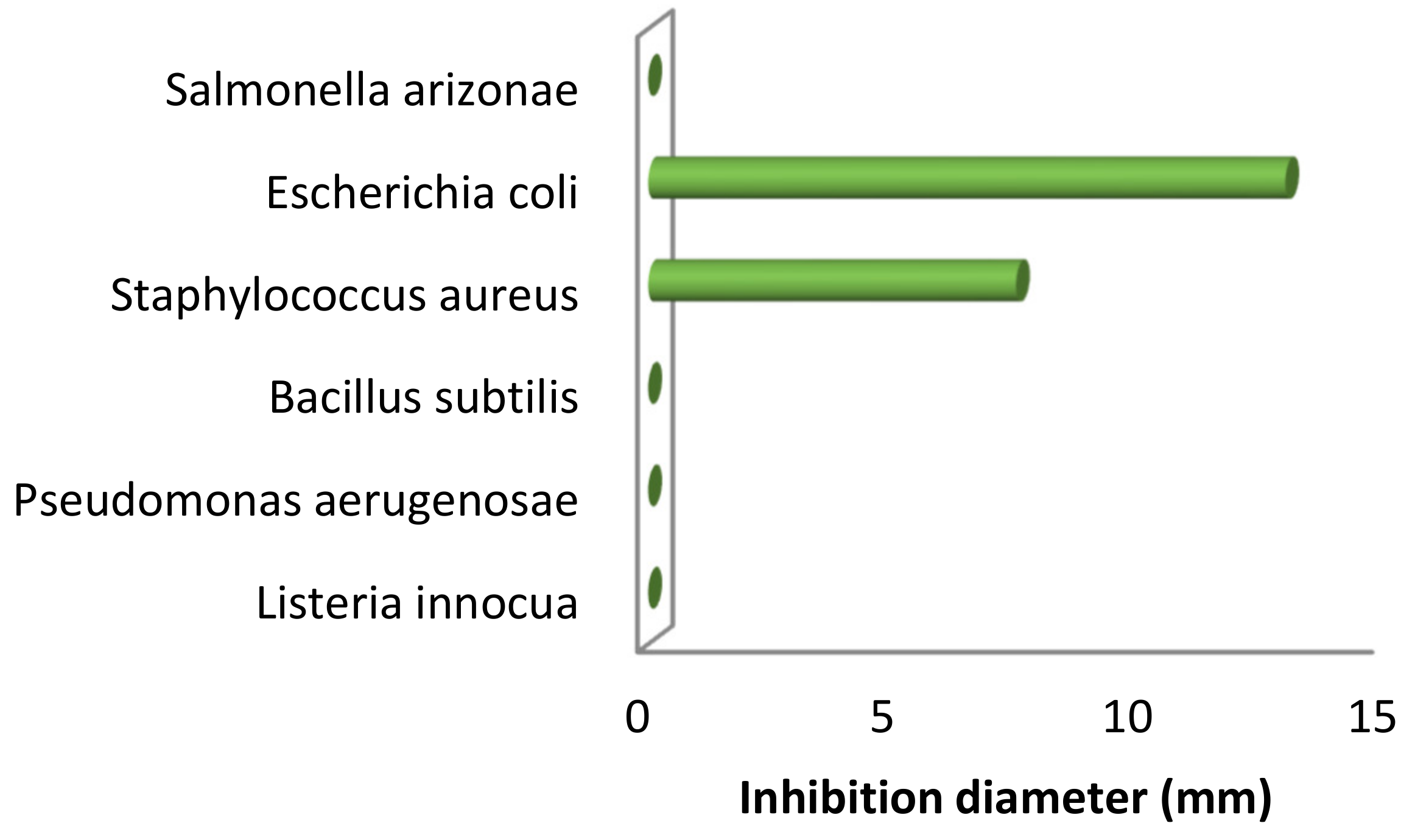
2.2.1. Antibacterial Activity
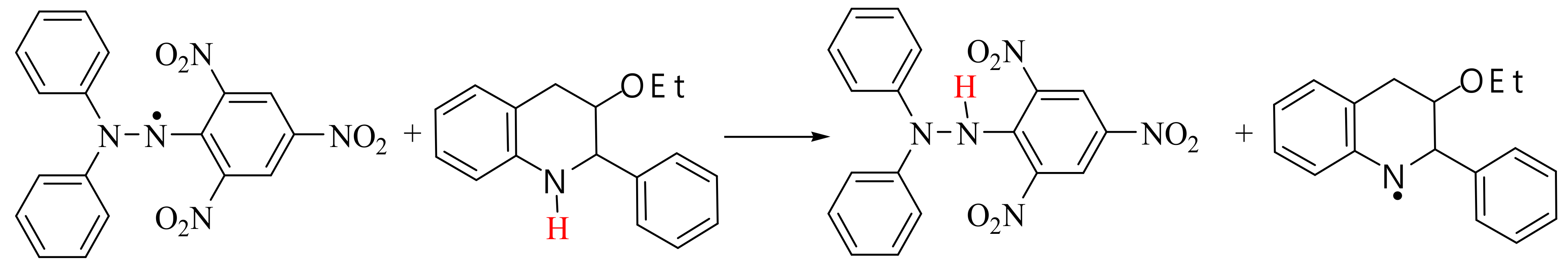
2.2.2. Antioxidant Activity
2.3. Computational Study
2.3.1. Methods and Models
2.3.2. Analysis of Reactivity Indices Derived from Conceptual DFT
Global Indices
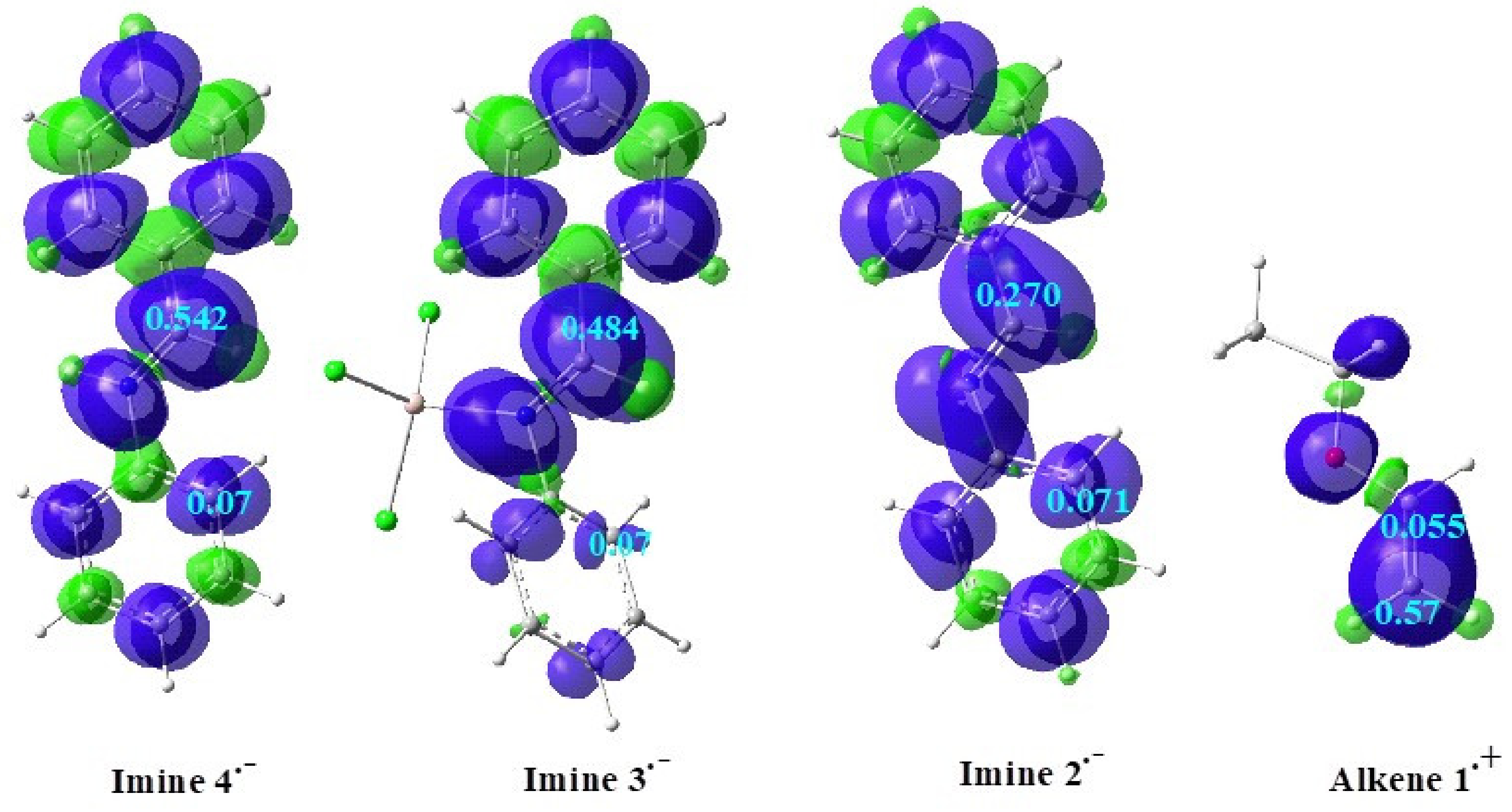
Local Indices
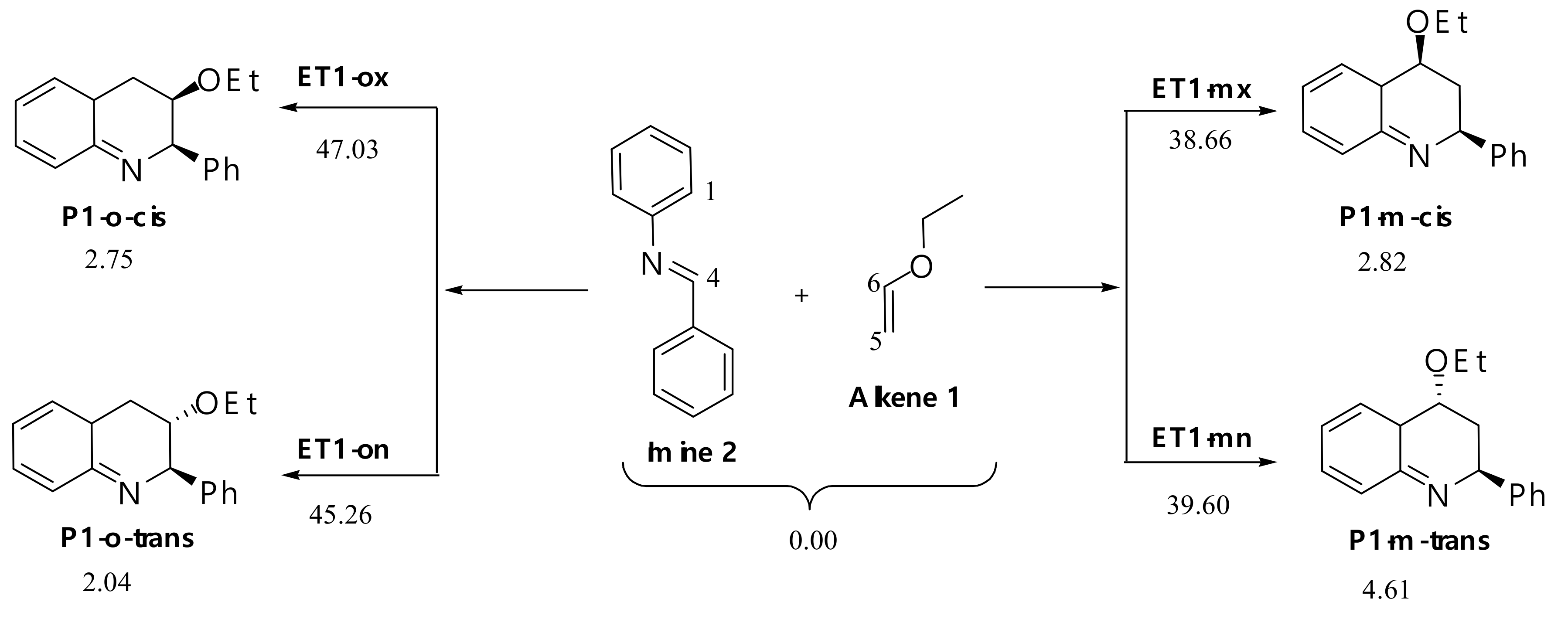
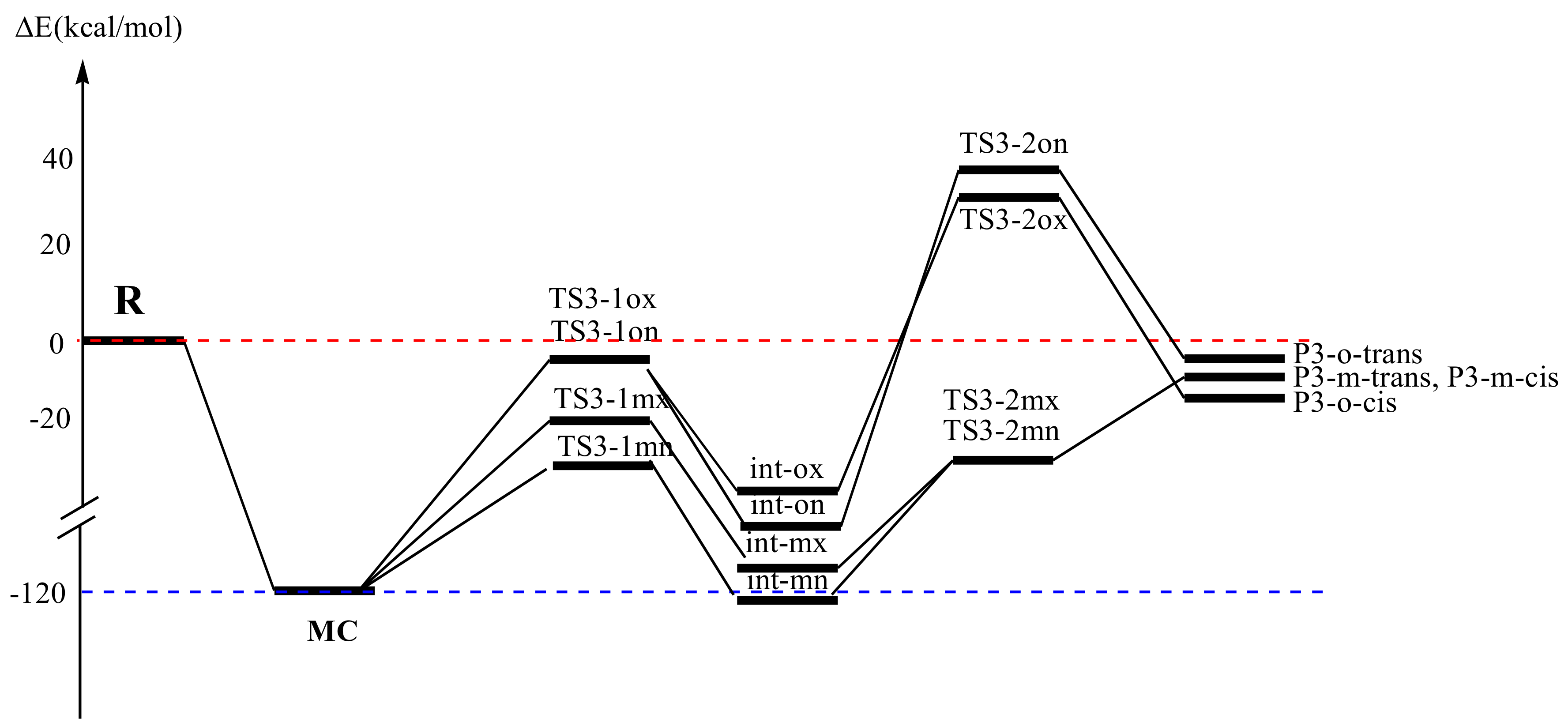
2.3.3. Energetic Aspects
Non-Catalyzed Reaction
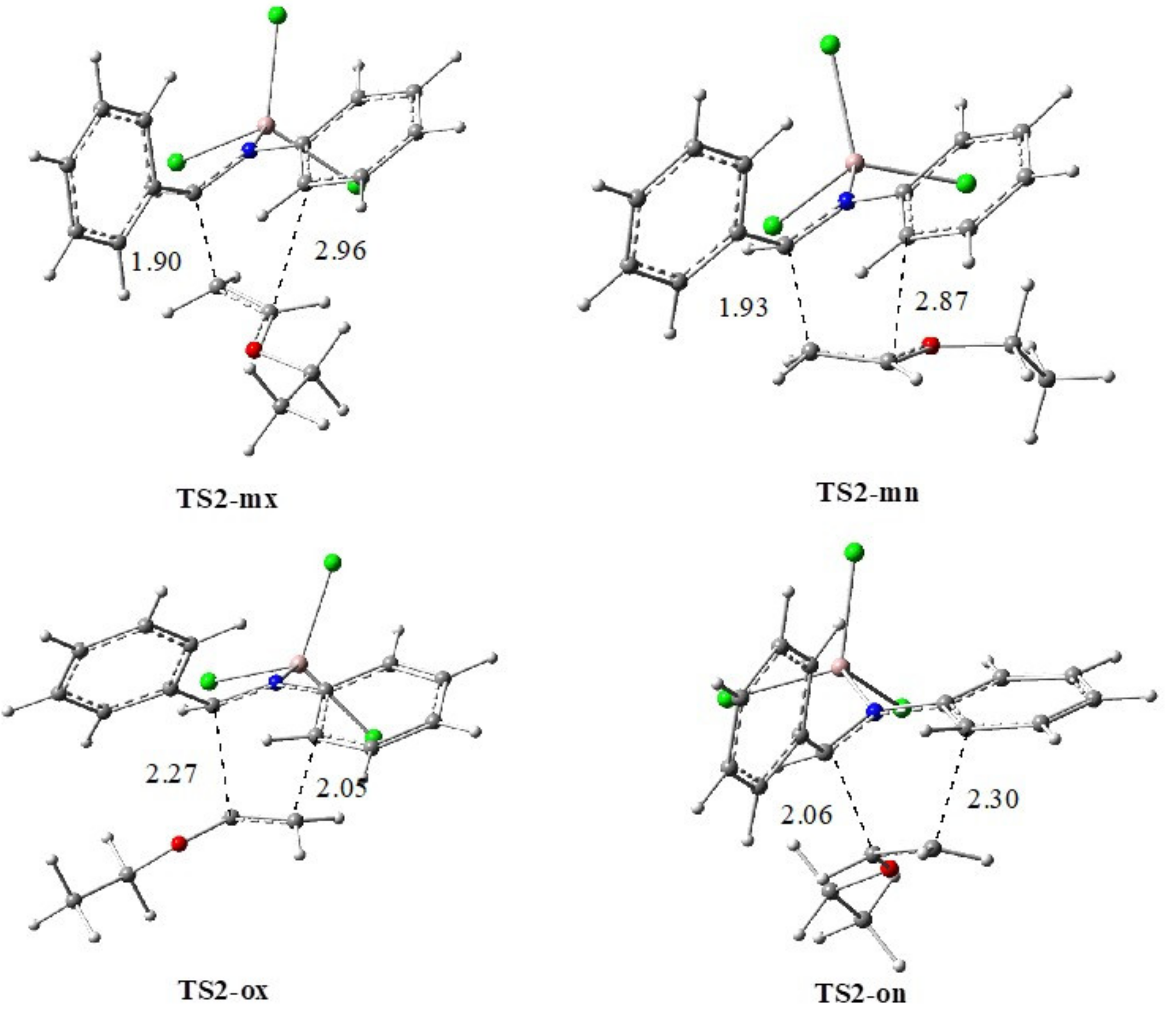
AlCl3 Catalyzed Reaction
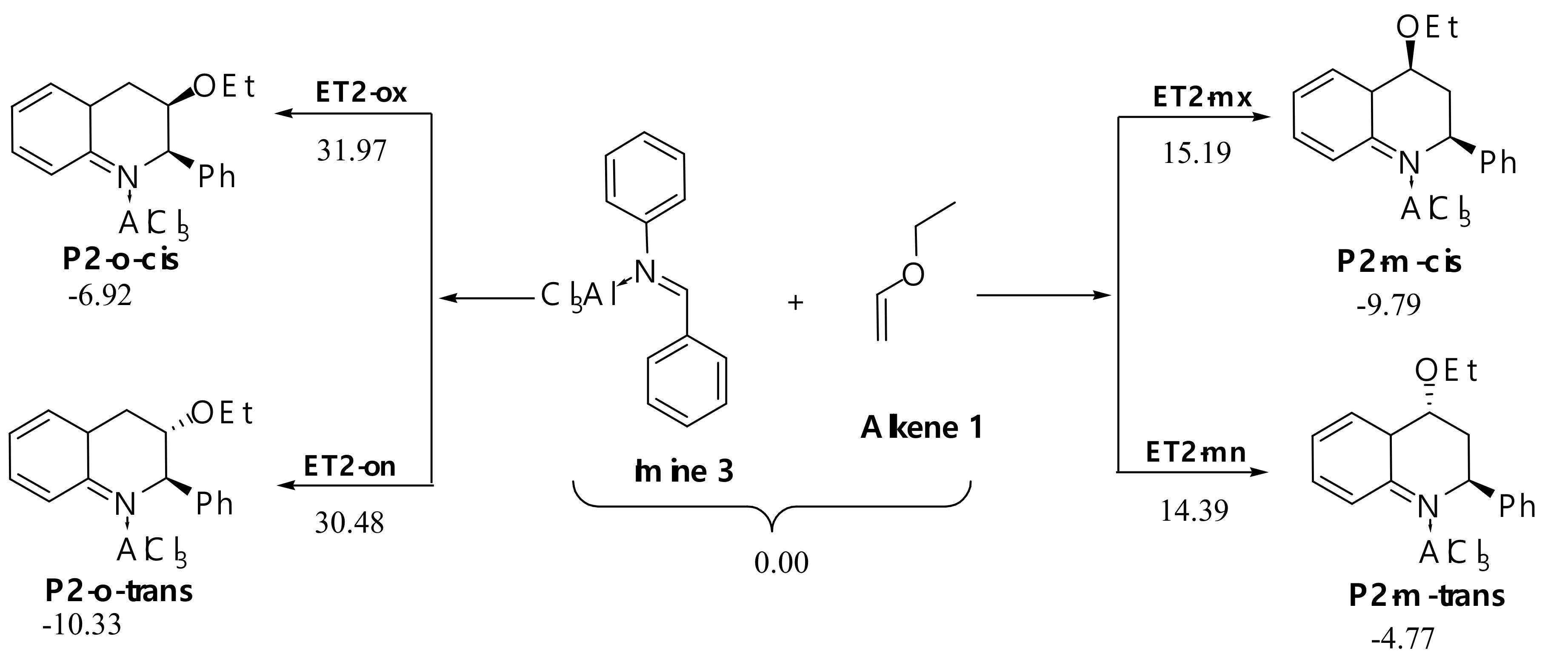
H+ Catalyzed Reaction
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vo, C.-V.T.; Bode, J.W. Synthesis of Saturated N-Heterocycles. J. Org. Chem. 2014, 79, 2809–2815. [Google Scholar] [CrossRef]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rano, T.A.; Sieber-McMaster, E.; Pelton, P.D.; Yang, M.; Demarest, K.T.; Kuo, G.-H. Design and synthesis of potent inhibitors of cholesteryl ester transfer protein (CETP) exploiting a 1,2,3,4-tetrahydroquinoline platform. Bioorg. Med. Chem. Lett. 2009, 19, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Nayak, T.K.; Burai, R.; Dennis, M.K.; Hathaway, H.J.; Sklar, L.A.; Prossnitz, E.R.; Arterburn, J.B. Synthesis and cha-racterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J. Med. Chem. 2010, 53, 1004–1014. [Google Scholar] [CrossRef] [Green Version]
- Caprio, V.; Guyen, B.; Opoku-Boahen, Y.; Mann, J.; Gowan, S.M.; Kelland, L.M.; Read, M.A.; Neidle, S. A novel inhibitor of human telomerase derived from 10H-indolo [3, 2-b] quinoline. Bioorg. Med. Chem. Lett. 2000, 10, 2063–2066. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Dinakaran, M.; Yogeeswari, P.; Sriram, D.; China, A.; Nagaraja, V. Synthesis and antimycobacterial activities of novel 6-nitroquinolone-3-carboxylic acids. Eur. J. Med. Chem. 2009, 44, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Wilson, R.W. Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry; Lippinicott Williams and Wilkins: Philadelphia, PA, USA, 1998; Volume 185. [Google Scholar]
- Guo, L.-J.; Wei, C.-X.; Jia, J.-H.; Zhao, L.-M.; Quan, Z.-S. Design and synthesis of 5-alkoxy-[1, 2, 4] triazolo [4, 3-a] quinoline derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2009, 44, 954–958. [Google Scholar] [CrossRef]
- Clemence, F.; Le Martret, O.; Delevallee, F.; Benzoni, J.; Jouanen, A.; Jouquey, S.; Mouren, M.; Deraedt, R. 4-Hydroxy-3-quinolinecarboxamides with antiarthritic and analgesic activities. J. Med. Chem. 1988, 31, 1453–1462. [Google Scholar] [CrossRef]
- Sircar, I.; Haleen, S.J.; Burke, S.E.; Barth, H. Synthesis and biological activity of 4-(diphenylmethyl)-alpha-[(4-quinolinyloxy)methyl]-1-piperazineethanol and related compounds. J. Med. Chem. 1992, 35, 4442–4449. [Google Scholar] [CrossRef]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels-Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rachwal, S.; Rachwal, B. Recent progress in the synthesis of 1, 2, 3, 4,-tetrahydroquinolines. Tetrahedron 1996, 52, 15031–15070. [Google Scholar] [CrossRef]
- Oikawa, H.; Tokiwano, T. Enzymatic catalysis of the Diels-Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 2004, 21, 321–352. [Google Scholar] [CrossRef]
- Lucchini, V.; Prato, M.; Scorrano, G.; Stivanello, M.; Valle, G. Acid-catalysed addition of N-aryl imines to dihydrofuran. Postu-lated dependence of the reaction mechanism on the relative face of approach of reactants. J. Chem. Soc. Perkin Trans. 1992, 2, 259–266. [Google Scholar] [CrossRef]
- Makioka, Y.; Shindo, T.; Taniguchi, Y.; Takaki, K.; Fujiwara, Y. Ytterbium (III) triflate catalyzed synthesis of quinoline derivatives from N-arylaldimines and vinyl ethers. Synthesis 1995, 1995, 801–804. [Google Scholar] [CrossRef]
- Liu, R.H.; Yu, X.; Hu, L.; Yu, N.F. Simple and practical synthesis of pyrano- and furano[3,2-c]-quinoline derivatives under non-Lewis acid catalysis. Chin. Chem. Lett. 2012, 23, 1027–1030. [Google Scholar] [CrossRef]
- Povarov, L.S. αβ-Unsaturated ethers and their analogues in reactions of diene synthesis. Russ. Chem. Rev. 1967, 36, 656–670. [Google Scholar] [CrossRef]
- Grieco, P.A.; Bahsas, A. Role reversal in the cyclocondensation of cyclopentadiene with heterodienophiles derived from aryl amines and aldehydes: Synthesis of novel tetrahydroquinolines. Tetrahedron Lett. 1988, 29, 5855–5858. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nagayama, S. A New Methodology for Combinatorial Synthesis. Preparation of Diverse Quinoline Derivatives Using a Novel Polymer-Supported Scandium Catalyst. J. Am. Chem. Soc. 1996, 118, 8977–8978. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.; Xie, M.; Sun, J. Lanthanide Chloride Catalyzed Imino Diels-Alder Reaction. One-Pot Synthesis of Pyrano [3, 2-c]-and Furo [3, 2-c] quinolines. J. Org. Chem. 1999, 64, 6462–6467. [Google Scholar] [CrossRef]
- Lavilla, R.; Bernabeu, M.C.; Carranco, I.; Díaz, J.L. Dihydropyridine-based multicomponent reactions. Efficient entry into new tetrahydroquinoline systems through Lewis acid-catalyzed formal [4+ 2] cycloadditions. Org. Lett. 2003, 5, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.A.; Batey, R.A. Lanthanide(III)-catalyzed multi-component aza-Diels-Alder reaction of aliphatic N-arylaldimines with cyclopentadiene. Tetrahedron Lett. 2003, 44, 7569–7573. [Google Scholar] [CrossRef]
- Batey, R.A.; Powell, D.A. Multi-component coupling reactions: Synthesis of a guanidine containing analog of the hexahydro-pyrrolo [3, 2-c] quinoline alkaloid martinelline. Chem. Commun. 2001, 22, 2362–2363. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Srinivas, R.; Madhuri, C.; Ramalingam, T. Lithium perchlorate/diethylether catalyzed aza-Diels-Alder reaction: An expeditious synthesis of pyrano, indeno quinolines and phenanthridines. Synlett 2001, 2001, 240–242. [Google Scholar] [CrossRef]
- Legros, J.; Crousse, B.; Ourevitch, M.; Bonnet-Delpon, D. Facile synthesis of tetrahydroquinolines and julolidines through mul-ticomponent reaction. Synlett 2006, 2006, 1899–1902. [Google Scholar] [CrossRef]
- Nacereddine, A.K.; Layeb, H.; Chafaa, F.; Yahia, W.; Djerourou, A.; Domingo, L.R. A DFT study of the role of the Lewis acid catalysts in the [3 + 2] cycloaddition reaction of the electrophilic nitrone isomer of methyl glyoxylate oxime with nucleophilic cyclopentene. RSC Adv. 2015, 5, 64098–64105. [Google Scholar] [CrossRef]
- Barama, L.; Bayoud, B.; Chafaa, F.; Nacereddine, A.K.; Djerourou, A. A mechanistic MEDT study of the competitive catalysed [4+2] and [2+2] cycloaddition reactions between 1-methyl-1-phenylallene and methyl acrylate: The role of Lewis acid on the mechanism and selectivity. Struct. Chem. 2018, 29, 1709–1721. [Google Scholar] [CrossRef]
- Bouacha, S.; Nacereddine, A.K.; Djerourou, A. A theoretical study of the mechanism, stereoselectivity and Lewis acid catalyst on the Diels-Alder cycloaddition between furan and activated alkenes. Tetrahedron Lett. 2013, 54, 4030–4033. [Google Scholar] [CrossRef]
- Yahia, W.; Nacereddine, A.K.; Liacha, M. Towards Understanding the Role of Lewis Acid on the Regioselectivity and Mechanism for the Acetylation Reaction of 2-benzoxazolinone with Acetyl Chloride: A DFT Study. Prog. React. Kinet. Mech. 2014, 39, 365–374. [Google Scholar] [CrossRef]
- Lachtar, Z.; Nacereddine, A.K. Understanding the origin of the enantioselectivity and the mechanism of the asymmetric re-duction of ketimine generated from acetophenone with oxazaborolidine catalyst. Struct. Chem. 2020, 31, 253–261. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, A.F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic che-mistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [Green Version]
- Gawroński, J.; Wascinska, N.; Gajewy, J. Recent Progress in Lewis Base Activation and Control of Stereoselectivity in the Additions of Trimethylsilyl Nucleophiles. Chem. Rev. 2008, 108, 5227–5252. [Google Scholar] [CrossRef]
- Nic, M.; Hovorka, L.; Jirat, J.; Kosata, B.; Znamenacek, J. IUPAC Compendium of Chemical Terminology—The Gold Book; International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2005. [Google Scholar]
- Schiff, H. Eine Reihe. Eur. J. Org. Chem. 1864, 131, 118–119. [Google Scholar]
- Layer, R.W. The Chemistry of Imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Posson, H.; Hurvois, J.-P.; Moinet, C. Imino Diels-Alder Reaction: Application to the Synthesis of Diverse Cyclopenta[c]Quinoline Derivatives. Synlett 2000, 2000, 209–212. [Google Scholar] [CrossRef]
- Kiselyov, A.S.; Smith, L.; Virgilio, A.; Armstrong, R.W. Immobilized aldehydes and olefins in the solid support synthesis of tetrahydroquinolines via a three component condensation. Tetrahedron 1998, 54, 7987–7996. [Google Scholar] [CrossRef]
- Seeley, H.W., Jr.; Van Demark, P.J. Microbes in Action. A Laboratory Manual of Microbiology; W. H. Freeman & Co.: San Francisco, CA, USA; London, UK, 1962. [Google Scholar]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural po-lyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.; Paganga, G.; Tijburg, L.; Bolwell, G.; Riceevans, C. Polyphenolic Flavanols as Scavengers of Aqueous Phase Radicals and as Chain-Breaking Antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian09; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A new mixing of Hartree-Fock and local density functional in atoms molecules and solids by the spin-density-functional formalism. Phys. Rev. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; PérezP, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef] [Green Version]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Gutiérrez, M.; Chafaa, F.; Nacereddine, A.K.; Djerourou, A.; Domingo, L.R. A DFT study of [3 + 2] cycloaddition reactions of an azomethine imine with N-vinyl pyrrole and N-vinyl tetrahydroindole. J. Mol. Graph. Model. 2016, 70, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Nacereddine, A.K.; Yahia, W.; Sobhi, C.; Djerourou, A. A theoretical study of the mechanism and stereoselectivity of the Diels–Alder cycloaddition between difluoro-2-methylencyclopropane and furan. Tetrahedron Lett. 2012, 53, 5784–5786. [Google Scholar] [CrossRef]
- Hellel, D.; Chafaa, F.; Nacereddine, A.K.; Djerourou, A.; Vrancken, E. Regio- and stereoselective synthesis of novel isoxazolidine heterocycles by 1,3-dipolar cycloaddition between C-phenyl-N-methylnitrone and substituted alkenes. Experimental and DFT investigation of selectivity and mechanism. RSC Adv. 2017, 7, 30128–30141. [Google Scholar] [CrossRef] [Green Version]
- Bayoud, B.; Barama, L.; Nacereddine, A.K.; Djerourou, A. Shedding light on the factors controlling the mechanism, selectivity and reactivity of the Diels-Alder reactions between substituted pyridinones and ethylenes: A MEDT study. Mol. Phys. 2021, 119, e1828635. [Google Scholar] [CrossRef]
- Nacereddine, A.K. A MEDT computational study of the mechanism, reactivity and selectivity of non-polar [3+2] cycloaddition between quinazoline-3-oxide and methyl 3-methoxyacrylate. J. Mol. Model. 2020, 26, 328. [Google Scholar] [CrossRef]
- Chermette, H. Chemical reactivity indexes in density functional theory. J. Comput. Chem. 1999, 20, 129–154. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. Theochem. 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Origin of the synchronicity in bond formation in polar Diels-Alder reactions: An ELF analysis of the reaction between cyclopentadiene and tetracyanoethylene. Org. Biomol. Chem. 2012, 10, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A DFT analysis of the participation of zwitterionic TACs in polar [3+2] cycloaddition reactions. Tetrahedron 2014, 70, 4519–4525. [Google Scholar] [CrossRef]
- Jasiński, R. First example of stepwise, zwitterionic mechanism for bicyclo [2.2. 1] hept-5-ene (norbornene) formation process catalyzed by the 1-butyl-3-methylimidazolium cations. Monatshefte Chem. Chem. Mon. 2016, 147, 1207–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. The mechanism of ionic Diels-Alder reactions. A DFT study of the oxa-Povarov reaction. RSC Adv. 2014, 4, 16567–16577. [Google Scholar] [CrossRef] [Green Version]



| Entry | Solvent | Catalyst | Temperature | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | diethylether | none | rt | 48 | no reaction |
| 2 | diethylether | AlCl3 | rt | 48 | 79 |
| 3 | ethanol | HCl | rt | 48 | 60 |
| 4 | ethanol/acetonitrile | CH3COOH | rt | 48 | 30 |
| Type of Bacteria | Inhibition Diameter (mm) |
|---|---|
| Listeria innocua CLIP 74915 | - |
| Pseudomonas aerugenosae ATCC 2592 | - |
| Bacillus subtilis ATCC 26023 | - |
| Staphylococcus aureus ATCC 29213 | 7.5 |
| Escherichiacoli ATCC 25922 | 13 |
| Salmonella arizonae CIP 81-3 | - |
| HOMO | LUMO | µ | η | ω | N | |
|---|---|---|---|---|---|---|
| Alkene 1 | −5.89 | 1.07 | −2.41 | 6.95 | 0.42 | 3.23 |
| Imine 2 | −5.91 | −1.57 | −3.74 | 4.34 | 1.61 | 3.21 |
| Imine 3 | −10.30 | −6.73 | −8.52 | 3.57 | 10.16 | −1.18 |
| Imine 4 | −7.15 | −2.90 | −5.03 | 4.25 | 2.97 | 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamri, S.; Heddam, A.; Kara, M.; Yahia, W.; Khorief Nacereddine, A. The Role of the Catalyst on the Reactivity and Mechanism in the Diels–Alder Cycloaddition Step of the Povarov Reaction for the Synthesis of a Biological Active Quinoline Derivative: Experimental and Theoretical Investigations. Organics 2021, 2, 57-71. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010006
Lamri S, Heddam A, Kara M, Yahia W, Khorief Nacereddine A. The Role of the Catalyst on the Reactivity and Mechanism in the Diels–Alder Cycloaddition Step of the Povarov Reaction for the Synthesis of a Biological Active Quinoline Derivative: Experimental and Theoretical Investigations. Organics. 2021; 2(1):57-71. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010006
Chicago/Turabian StyleLamri, Soumia, Affaf Heddam, Meriem Kara, Wassila Yahia, and Abdelmalek Khorief Nacereddine. 2021. "The Role of the Catalyst on the Reactivity and Mechanism in the Diels–Alder Cycloaddition Step of the Povarov Reaction for the Synthesis of a Biological Active Quinoline Derivative: Experimental and Theoretical Investigations" Organics 2, no. 1: 57-71. https://0-doi-org.brum.beds.ac.uk/10.3390/org2010006






