Development of Efficient and Selective Processes for the Synthesis of Commercially Important Chlorinated Phenols
Abstract
:1. Introduction
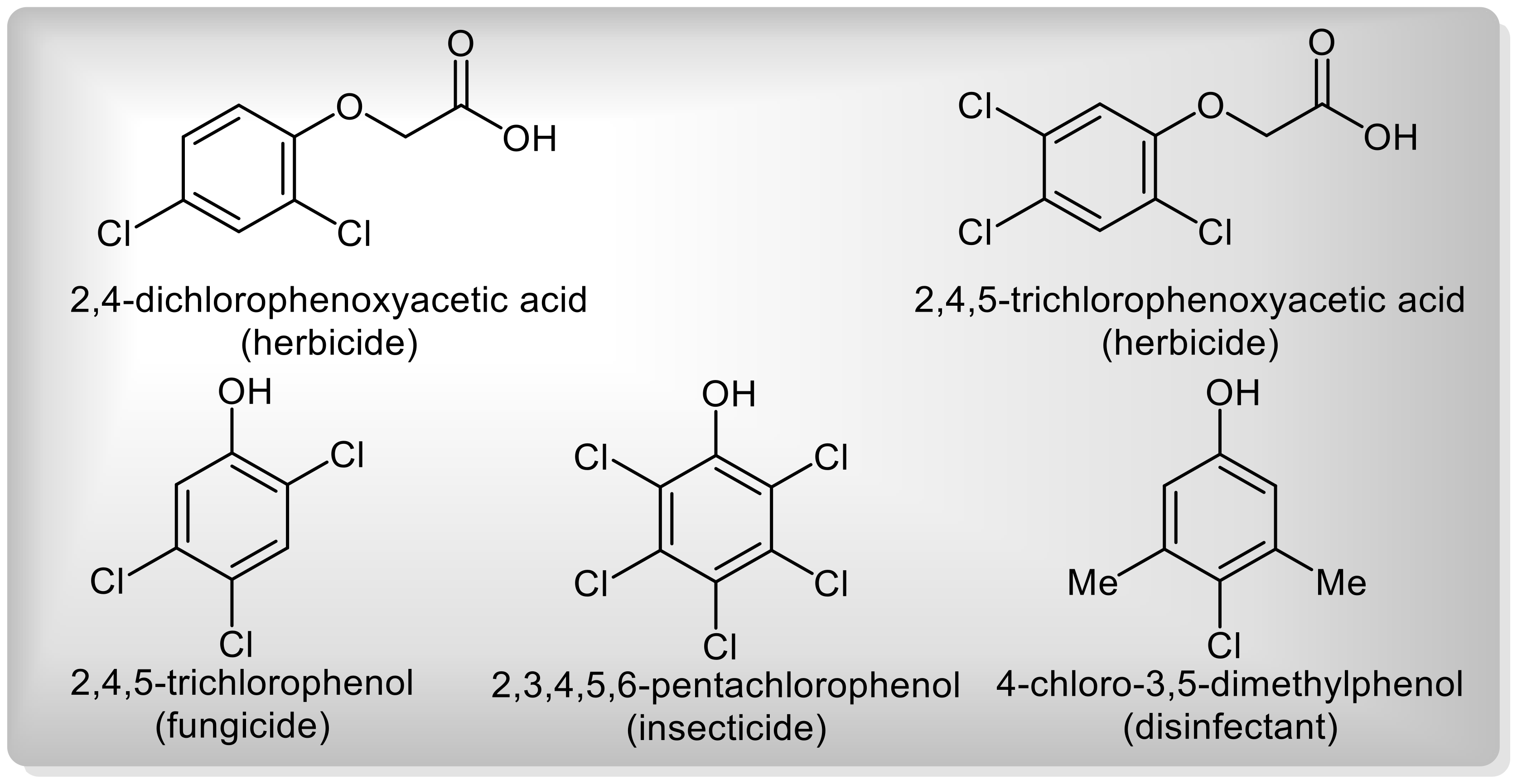
1.1. Commercial Importance of Chlorinated Phenols
1.2. Research Group Background in Selective Aromatic Substitution Reactions
2. The Use of Structured Solids to Influence the Halogenation Reactions of Aromatic Compounds
3. Early Studies of Halogenation of Phenols
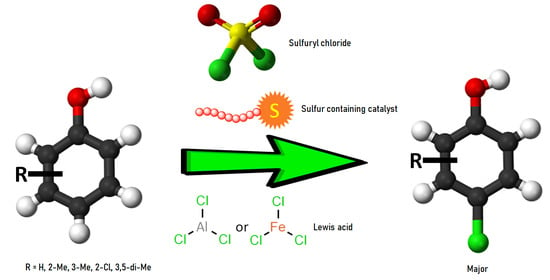
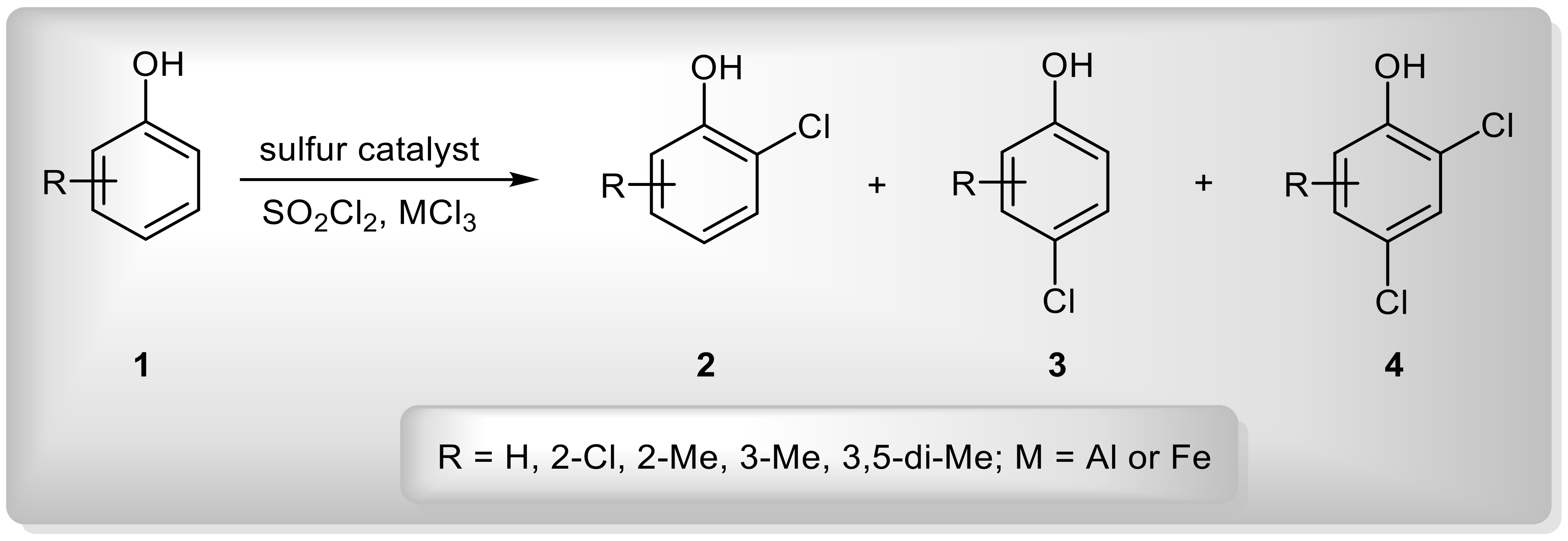
4. Development of a Successful Approach to para-Chlorination of Phenols Using Sulphur Compounds as Catalysts
4.1. Results with Simple Dialkyl Sulphides and Some Cyclic Analogues
4.2. Results with Dithiaalkanes 10 [(H(CH2)mS(CH2)nS(CH2)mH] and Related Compounds
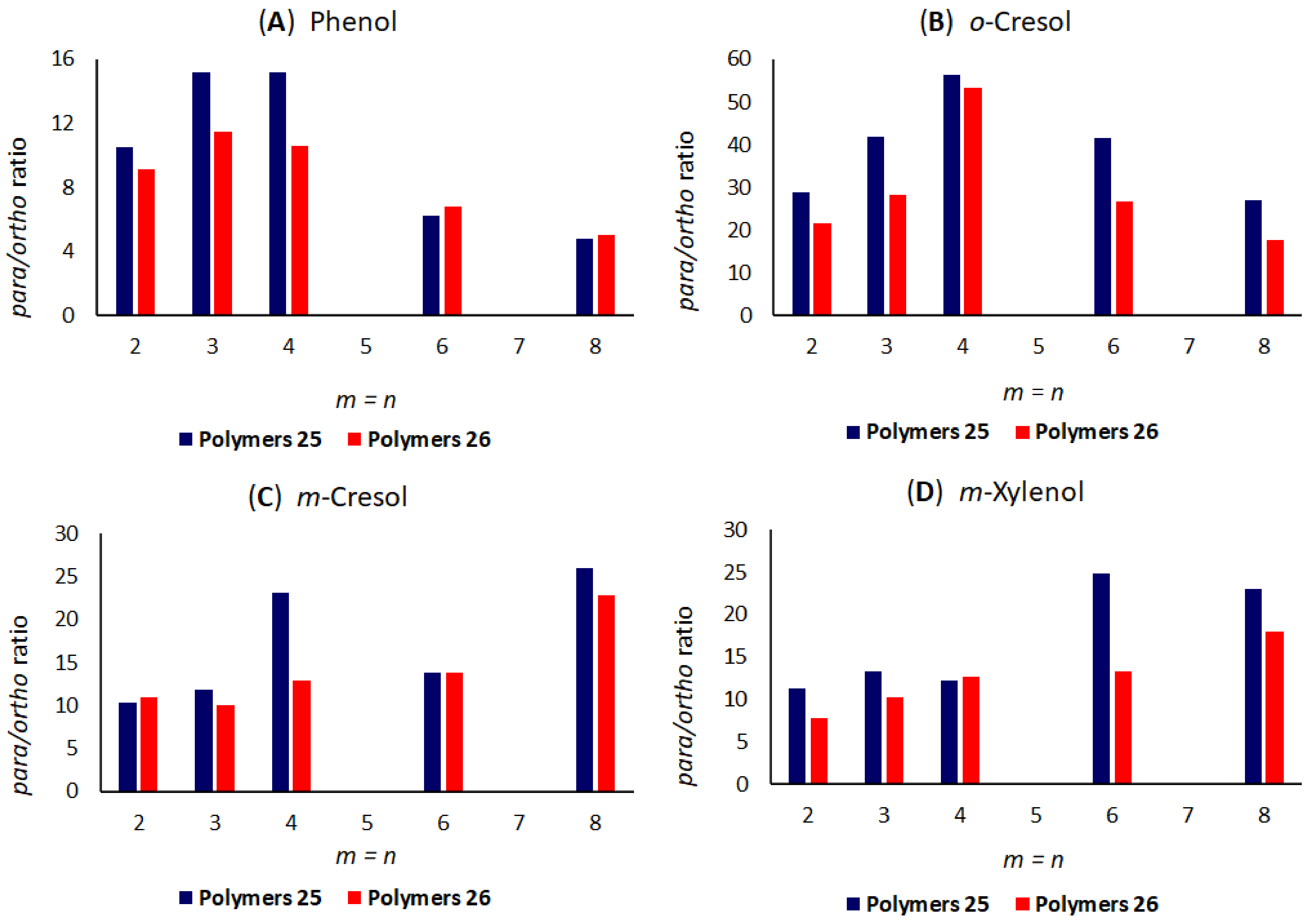
4.3. Results with Polymeric Compounds Containing Multiple Sulphur Atoms
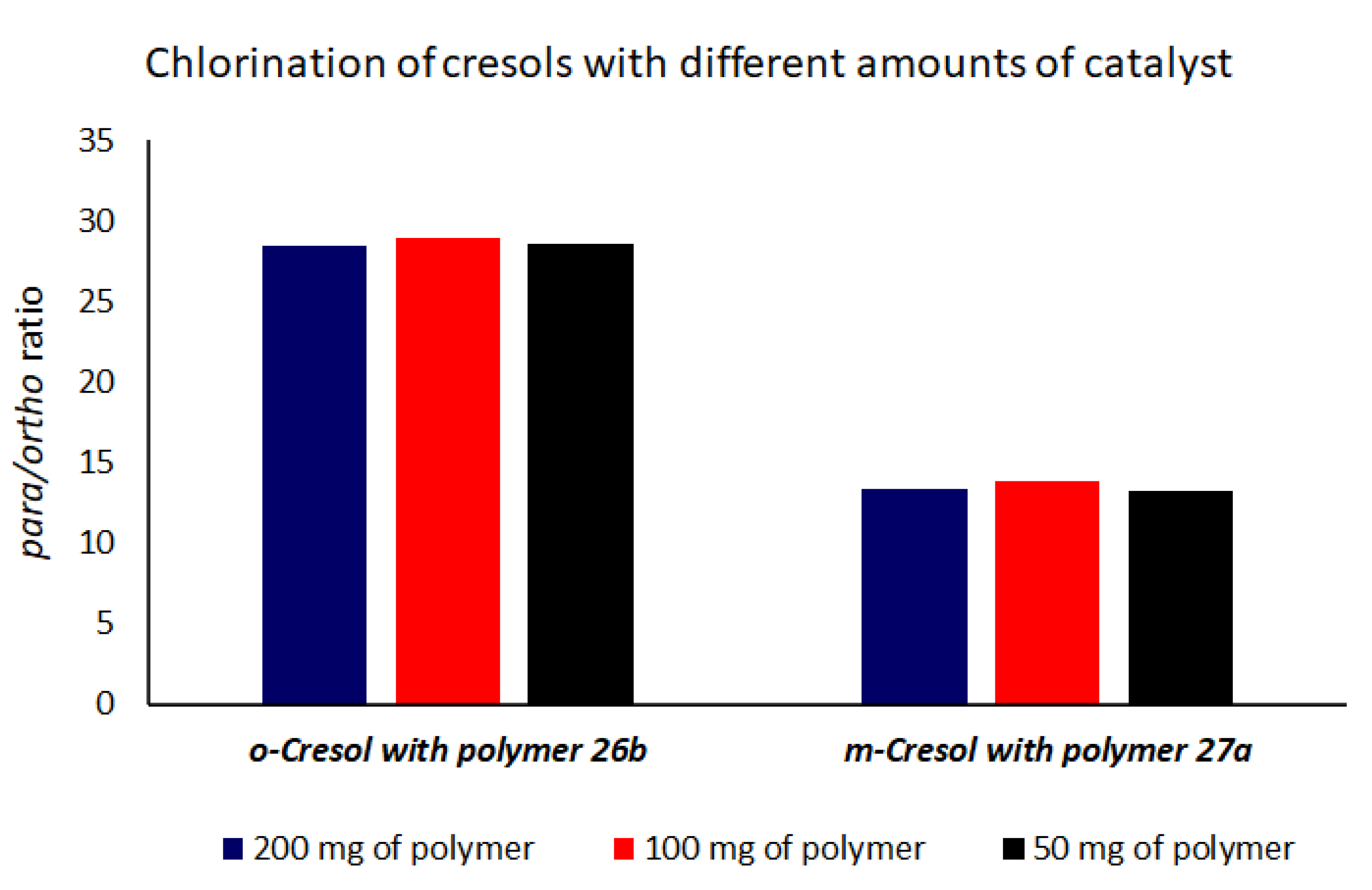
4.4. Results with Poly(alkylene sulphide)s
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palleros, D.R. Experimental Organic Chemistry; John Wiley Sons: New York, NY, USA, 2000. [Google Scholar]
- Chikhradze, N.; Nadirashvili, M.; Khomeriki, S.; Varshanidze, I. The synthesis of phenyl acetylene phenols for development of new explosives. IOP Conf. Ser. Earth Environ. Sci. 2017, 95, 042030. [Google Scholar] [CrossRef]
- Khabarov, Y.G.; Patrakeev, A.A.; Veshnyakov, V.A.; Kosyakov, D.S.; Ul’yanovskii, N.V.; Garkotin, A.Y. One-step synthesis of picric acid from phenol. Org. Prep. Proced. Int. 2017, 49, 178–181. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfia, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [Green Version]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Hesse, W. Phenolic Resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- Cook, A.M. Phenolic disinfectants. J. Pharm. Pharmacol. 1960, 12, 19T–28T. [Google Scholar] [CrossRef]
- Reddy, V.P.; Prakash, G.K.S. Electrophilic Reactions of Phenols. In PATAI’s Chemistry of Functional Groups; John Wiley Sons: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Wiley-VCH. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Fagan, K.; John, K.P. The effect of the phenoxyacetic acid herbicides 2,4,5-trichlorophenoxyacetic acid and 2,4-dichlorophenoxyacetic acid as ascertained by direct experimentation. Residue Rev. 1984, 92, 29–58. [Google Scholar] [CrossRef]
- Favaro, G.; De Leo, D.; Pastore, P.; Magno, F.; Ballardin, A. Quantitative determination of chlorophenols in leather by pressurized liquid extraction and liquid chromatography with diode-array detection. J. Chromatogr. A 2008, 1177, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Model Formulary 2008; Stuart, M.C., Kouimtzi, M., Hill, S.R., Eds.; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Griffiths, C.; Barker, J.; Bleiker, T.O.; Chalmers, R.; Creamer, D. Rook’s Textbook of Dermatology, 9th ed.; John Wiley Sons: Oxford, UK, 2017. [Google Scholar]
- Digison, M.B. A review of anti-septic agents for pre-operative skin preparation. Plast. Surg. Nurs. 2007, 27, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hiti, G.A.; Smith, K.; Hegazy, A.S. Catalytic, green and regioselective Friedel-Crafts acylation of simple aromatics and heterocycles over zeolites. Curr. Org. Chem. 2015, 19, 585–598. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A. Use of zeolites for green and para-selective electrophilic aromatic substitution reactions. Green Chem. 2011, 13, 1579–1608. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; El-Hiti, G.A. Regioselective electrophilic aromatic substitution reactions over reusable zeolites. Curr. Org. Chem. 2006, 10, 1603–1625. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; El-Hiti, G.A. Regioselective control of electrophilic aromatic substitution reactions. Curr. Org. Synth. 2004, 1, 253–274. [Google Scholar] [CrossRef]
- Smith, K.; Al-Khalaf, A.K.H.; El-Hiti, G.A.; Pattisson, S. Highly regioselective di-tert-amylation of naphthalene over reusable HM zeolite catalyst. Green Chem. 2012, 14, 1103–1110. [Google Scholar] [CrossRef]
- Smith, K.; Roberts, S.D.; El-Hiti, G.A. Study of regioselective dialkylation of naphthalene in the presence of reusable zeolite catalysts. Org. Biomol. Chem. 2003, 1, 1552–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; El-Hiti, G.A.; Jayne, A.J.; Butters, M. Acetylation of aromatic ethers using acetic anhydride over solid acid catalysts in a solvent-free system. Scope of the reaction for substituted ethers. Org. Biomol. Chem. 2003, 1, 1560–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; El-Hiti, G.A.; Jayne, A.J.; Butters, M. Acylation of aromatic ethers over solid acid catalysts: Scope of the reaction with more complex acylating agents. Org. Biomol. Chem. 2003, 1, 2321–2325. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Ewart, G.M.; El-Hiti, G.A.; Randles, K.R. Study of regioselective methanesulfonylation of simple aromatics with methanesulfonic anhydride in the presence of reusable zeolite catalysts. Org. Biomol. Chem. 2004, 2, 3150–3154. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Alotaibi, M.H.; El-Hiti, G.A. Regioselective nitration of 2- and 4-nitrotoluenes over systems comprising nitric acid, an acid anhydride and a zeolite. Arkivoc 2014, 2014, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Alotaibi, M.H.; El-Hiti, G.A. Regioselective dinitration of simple aromatics over zeolite Hβ/nitric acid/acid anhydride systems. Arkivoc 2014, 2014, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Alotaibi, M.H.; El-Hiti, G.A. Highly regioselective dinitration of toluene over zeolite Hβ. J. Catal. 2013, 297, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Ajarim, M.D.; El-Hiti, G.A. Regioselective nitration of deactivated aromatics using acyl nitrates over reusable acidic zeolite catalysts. Catal. Lett. 2010, 134, 270–278. [Google Scholar] [CrossRef]
- Smith, K.; Liu, S.; El-Hiti, G.A. Regioselective mononitration of simple aromatic compounds under mild conditions in ionic liquid. Ind. Eng. Chem. Res. 2005, 44, 8611–8615. [Google Scholar] [CrossRef]
- Smith, K.; Musson, A.; DeBoos, G.A. A novel method for the nitration of simple aromatic compounds. J. Org. Chem. 1998, 63, 8448–8454. [Google Scholar] [CrossRef]
- Smith, K.; Musson, A.; DeBoos, G.A. Superior methodology for the nitration of simple aromatic compounds. Chem. Commun. 1996, 469–470. [Google Scholar] [CrossRef]
- Smith, K.; Butters, M.; Paget, W.E.; Nay, B. New reagent systems for electrophilic chlorination of aromatic compounds: Organic chlorine-containing compounds in the presence of silica. Synthesis 1985, 1155–1156. [Google Scholar] [CrossRef]
- Mistry, A.G.; Smith, K.; Bye, M.R. A superior synthetic method for the bromination of indoles and benzimidazoles. Tetrahedron Lett. 1986, 27, 1051–1054. [Google Scholar] [CrossRef]
- Smith, K.; James, D.M.; Mistry, A.G.; Bye, M.R.; Faulkner, D.J. A new method for bromination and polybromination of carbazoles, β-carbolines and iminodibenzyls by using N-bromosuccinimide and silica gel. Tetrahedron 1992, 48, 7479–7488. [Google Scholar] [CrossRef]
- Jigajinni, V.B.; Paget, W.E.; Smith, K. The synthesis of alkyl chlorides via reaction of trialkylboranes with dichloramine-T or N,N-dichlorourethane. J. Chem. Res. 1981, 376–377. [Google Scholar]
- Mistry, A.G.; Smith, K.; Bye, M.R. Solid Supported Halogenations: A Novel Method for Bromination of Heterocycles. In Bromine Compounds: Chemistry and Applications; Price, D., Iddon, B., Wakefield, B.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 277–295. [Google Scholar]
- Smith, K. Controlled Bromination with the Help of Microporous Solids. In Advances in Organobromine Chemistry I; Desmurs, J.-R., Gérard, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 5–21. [Google Scholar]
- Smith, K.; Butters, M.; Paget, W.E.; Goubet, D.; Fromentin, E.; Nay, B. Highly selective monochlorination of aromatic compounds under mild conditions by tert-butyl hypochlorite in the presence of zeolites. Green Chem. 1999, 1, 83–90. [Google Scholar] [CrossRef]
- Smith, K.; He, P.; Taylor, A. Highly selective liquid phase para-bromination of phenyl acetate catalysed by zeolites and metal acetates. Green Chem. 1999, 1, 35–38. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Hammond, M.E.W.; Bahzad, D.; Li, Z.; Siquet, C. Highly efficient and selective electrophilic and free radical catalytic bromination reactions of simple aromatic compounds in the presence of reusable zeolites. J. Chem. Soc. Perkin Trans. 1 2000, 2745–2752. [Google Scholar] [CrossRef]
- Smith, K.; Bahzad, D. Highly efficient para-Selective bromination of simple aromatic substrates by means of bromine and a reusable zeolite. Chem. Commun. 1996, 467–468. [Google Scholar] [CrossRef]
- Maloney, V.; Szczepansk, Z.; Smith, K. Introduction of a simple experiment for the undergraduate organic chemistry laboratory demonstrating the Lewis acid and shape selective properties of zeolites. J. Chem. Ed. 2017, 94, 1343–1346. [Google Scholar] [CrossRef]
- Smith, K.; Butters, M.; Nay, B. High ortho-selectivity in the chlorination of phenols with N-chlorodialkylamines in the presence of silica. Tetrahedron Lett. 1988, 29, 1319–1322. [Google Scholar] [CrossRef]
- Smith, K.; Ajarim, M.D.; El-Hiti, G.A.; Peters, C. Catalytic mononitration of phenol using iso-propyl nitrate over zeolite catalysts. Topics Catal. 2009, 52, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Gnaim, J.M.; Sheldon, R.A. Shape-selective para-chlorination of phenol using sulfuryl chloride with the aid of microporous catalysts. Tetrahedron Lett. 2004, 45, 9397–9399. [Google Scholar] [CrossRef]
- Smith, K.; James, D.M.; Matthews, I.; Bye, M.R. Selective para-bromination of phenols via a regenerable polymer-bound tetraalkylammonium tribromide. J. Chem. Soc. Perkin Trans. 1 1992, 1877–1878. [Google Scholar] [CrossRef]
- Sullivan, J.D. Para-Halogenation of phenols. Chem. Abstr. 1957, 51, 77109. [Google Scholar]
- Nishihara, A.; Kato, H. Chlorination of phenols. Chem. Abstr. 1974, 81, 120196. [Google Scholar]
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 4th ed.; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Xin, H.; Yang, S.; An, B.; An, Z. Selective water-based oxychlorination of phenol with hydrogen peroxide catalysed by manganous sulfate. RSC Adv. 2017, 7, 13467–13472. [Google Scholar] [CrossRef] [Green Version]
- Nahide, P.D.; Ramadoss, V.; Juárez-Ornelas, K.A.; Satkar, Y.; Ortiz-Alvarado, R.; Cervera-Villanueva, J.M.; Alonso-Castro, Á.J.; Zapata-Morales, J.R.; Ramírez-Morales, M.A.; Ruiz-Padilla, A.J.; et al. In situ formed IIII-based reagent for the electrophilic ortho-chlorination of phenols and phenol ethers: The use of PIFA-AlCl3 System. Eur. J. Org. Chem. 2018, 2018, 485–493. [Google Scholar] [CrossRef]
- Xiong, X.D.; Yeung, Y.-Y. Ammonium salt-catalyzed highly practical ortho-selective monohalogenation and phenylselenation of phenols: Scope and applications. ACS Catal. 2018, 8, 4033–4043. [Google Scholar] [CrossRef]
- Bugnet, E.A.; Brough, A.R.; Greatrex, R.; Kee, T.P. On the para-selective chlorination of ortho-cresol. Tetrahedron 2002, 58, 8059–8065. [Google Scholar] [CrossRef]
- Watson, W.D. Chlorination with sulfuryl chloride. Chem. Abstr. 1976, 84, 43612. [Google Scholar]
- Watson, W.D. The regioselective para chlorination of 2-methylphenol. Tetrahedron Lett. 1976, 17, 2591–2594. [Google Scholar] [CrossRef]
- Watson, W.D. Regioselective para-chlorination of activated aromatic compounds. J. Org. Chem. 1985, 50, 2145–2148. [Google Scholar] [CrossRef]
- Binns, J.S.; Braithwaite, M.J. p-Chlorophenol. Chem. Abstr. 1978, 88, 120784. [Google Scholar]
- Ogata, Y.; Kimura, M.; Kondo, Y.; Katoh, H.; Chen, F.-C. Orientation in the chlorination of phenol and of anisole with sodium and t-butyl hypochlorites in various solvents. J. Chem. Soc. Perkin. Trans. 2 1984, 451–453. [Google Scholar] [CrossRef]
- Olah, G.A.; Ohannesian, L.; Arvanaghi, M. Synthetic methods and reactions; 127. Regioselective para halogenation of phenols, phenol ethers and anilines with halodimethylsulfonium halides. Synthesis 1986, 868–870. [Google Scholar] [CrossRef]
- Tzimas, M.; Smith, K.; Brown, C.M.; Payne, K. Chlorination of aromatic compounds and catalysts therefor. Chem. Abstr. 1998, 129, 260219. [Google Scholar]
- Smith, K.; Tzimas, M.; Brown, C.M.; Payne, K. Dialkyl sulfides as selective catalysts for the chlorination of phenols. Sulfur Lett. 1999, 22, 89–101. [Google Scholar]
- Tzimas, M. Selective Control of Chlorination of Phenols. Ph.D. Thesis, University of Wales Swansea, Swansea, UK, 1995. [Google Scholar]
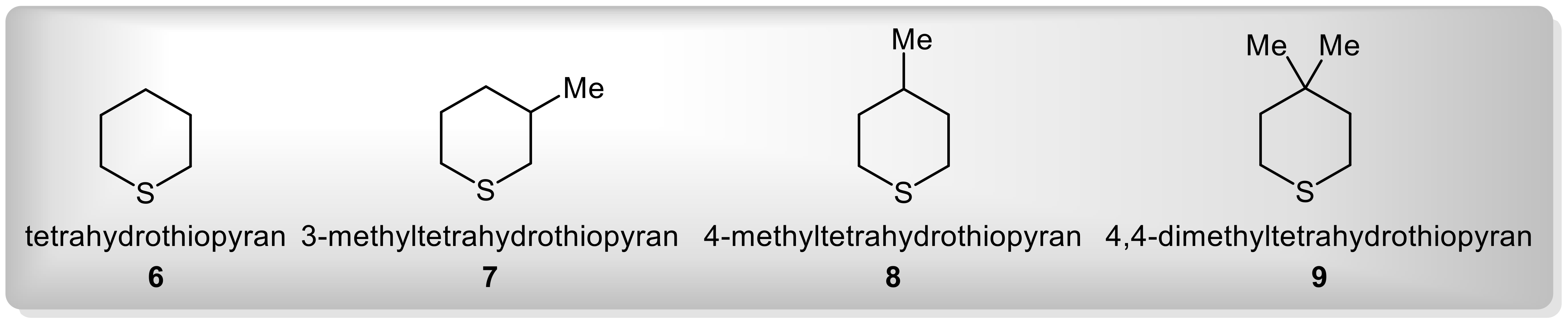
- Smith, K.; Williams, D.; El-Hiti, G.A. Regioselective chlorination of phenols in the presence of tetrahydrothiopyran derivatives. J. Sulfur Chem. 2019, 40, 529–538. [Google Scholar] [CrossRef]
- Tzimas, M.; Smith, K.; Brown, C.M.; Payne, K. Chlorination of aromatic compounds and catalysts therefor. Chem. Abstr. 1998, 129, 275696. [Google Scholar]
- Smith, K.; Tzimas, M.; Brown, C.M.; Payne, K. Dithiaalkanes and modified Merrifield resins as selective catalysts for the chlorination of phenols. Sulfur Lett. 1999, 22, 103–123. [Google Scholar]
- Smith, K.; Al-Zuhairi, A.J.; Elliot, M.C.; El-Hiti, G.A. Regioselective synthesis of important chlorophenols in the presence of methylthioalkanes with remote SMe, OMe or OH substituents. J. Sulfur Chem. 2018, 39, 607–621. [Google Scholar] [CrossRef]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Vo, C.D.; Kilcher, G.; Tirelli, N. Polymers and sulfur: What are organic polysulfides good for? Preparative strategies and biological applications. Macromol. Rapid Commun. 2009, 30, 299–315. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Al-Zuhairi, A.J. The synthesis of polymeric sulfides by reaction of dihaloalkanes with sodium sulfide. J. Sulfur Chem. 2011, 32, 521–531. [Google Scholar] [CrossRef]
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. Previously unpublished results from the authors’ group. 2020. [Google Scholar]
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. The use of polymeric sulfides as catalysts for the para-regioselective chlorination of phenol and 2-chlorophenol. J. Sulfur Chem. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. para-Selective chlorination of cresols and m-xylenol using sulfuryl chloride in the presence of poly(alkylene sulfide)s. J. Sulfur Chem. 2020, 41, 345–356. [Google Scholar] [CrossRef]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A. Previously unpublished results from the authors’ group. 2012. [Google Scholar]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)–borane: Reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]




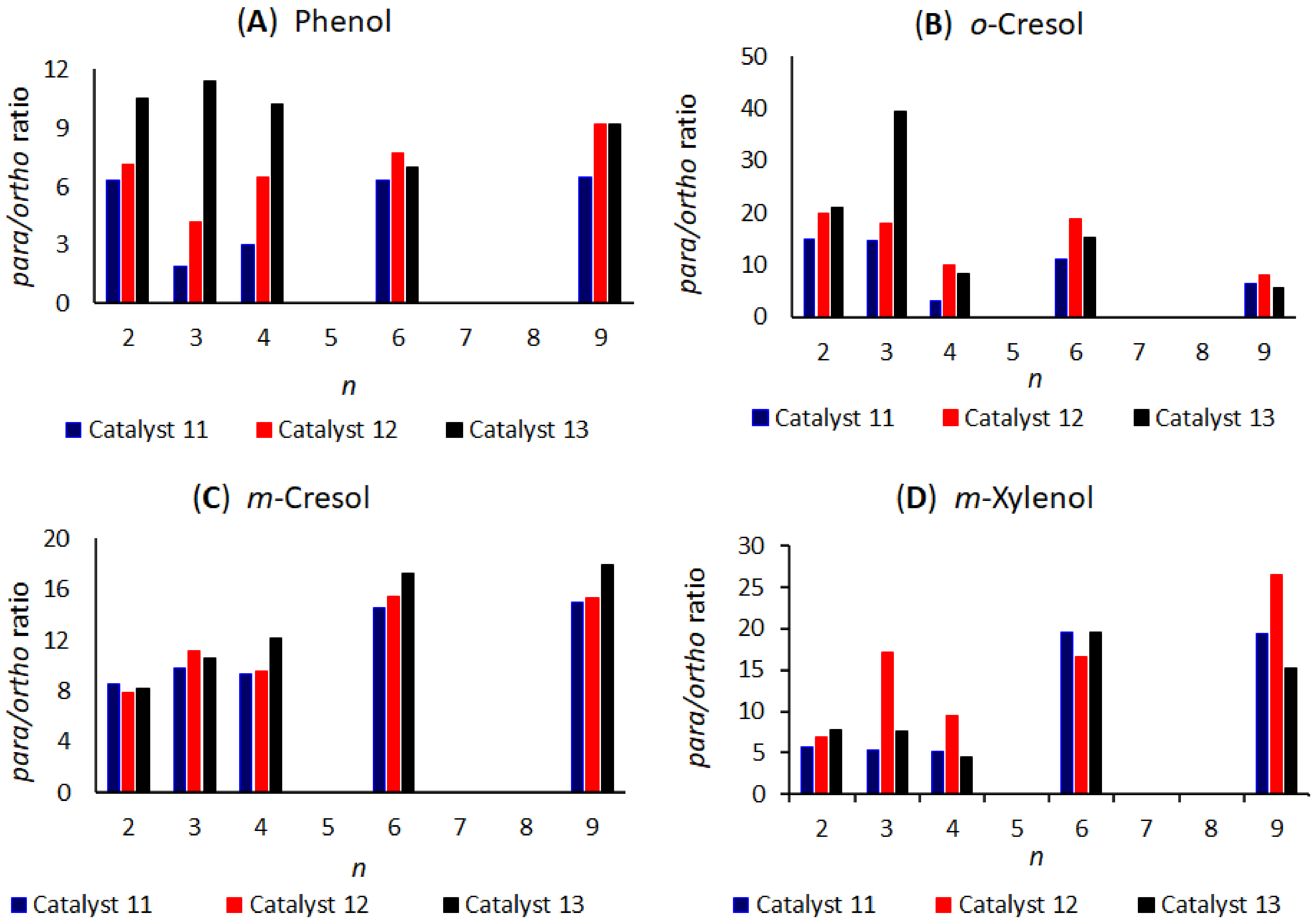
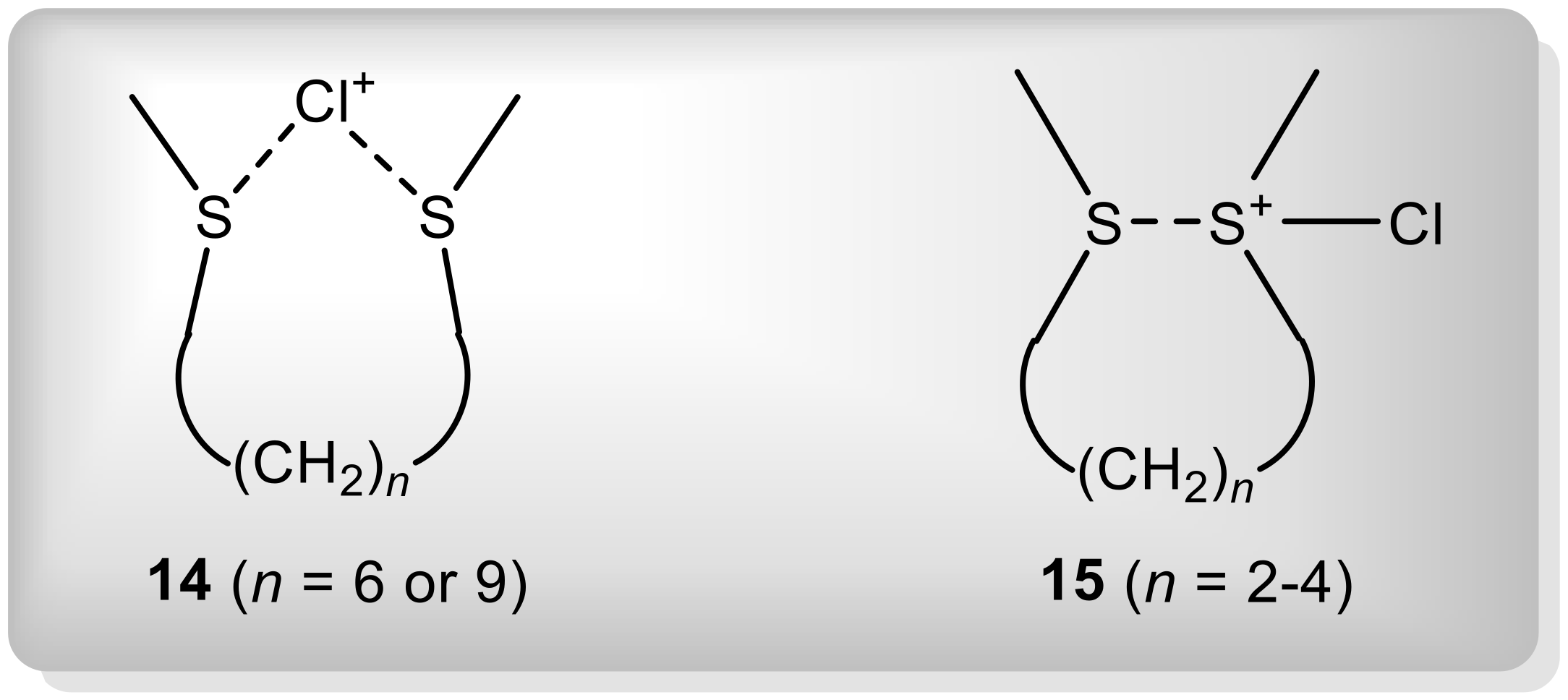
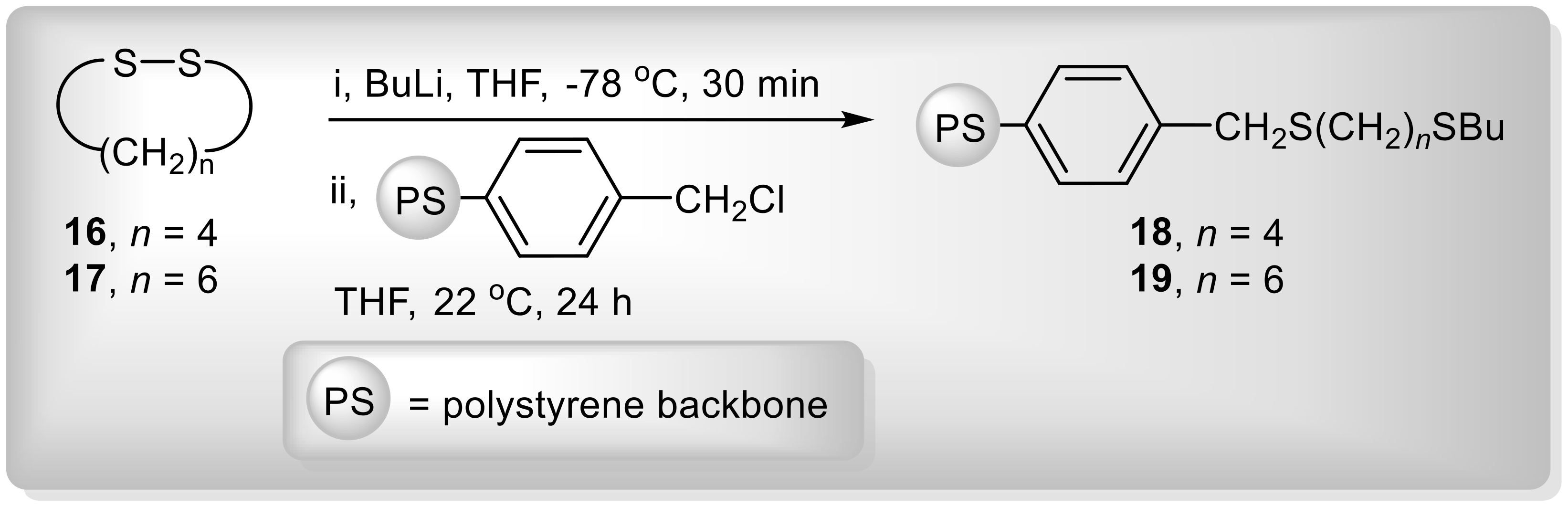
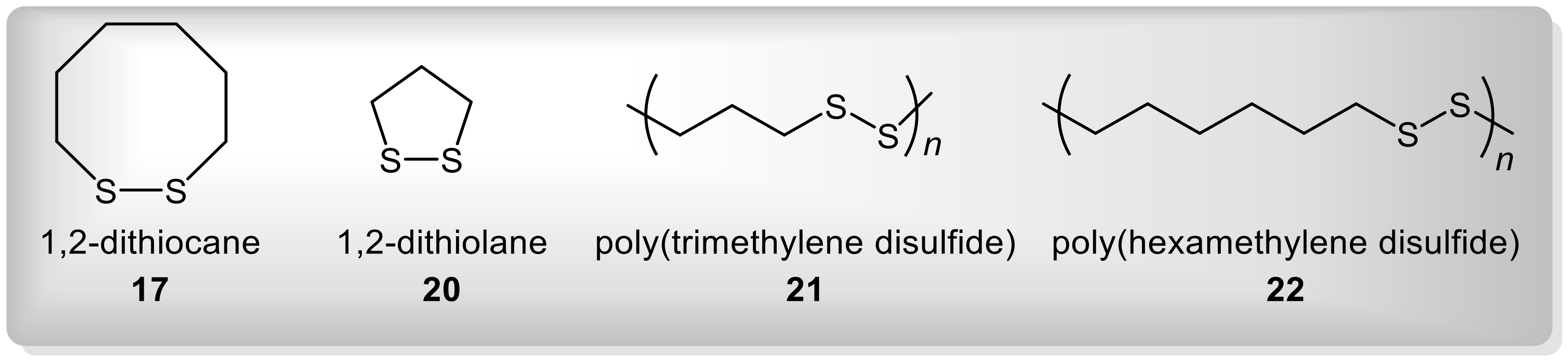



| Entry | Substrate | R1 | R2 | R1R2S (mmol) | AlCl3 (mmol) | Temperature (°C) | 3:2 Ratio b |
|---|---|---|---|---|---|---|---|
| 1 | Phenol | — | — | — | — | 35 | 4.8 |
| 2 | Phenol | Me | Me | 2.7 | — | 20 | 2.4 |
| 3 | Phenol | n-Bu | n-Bu | 2.7 | — | 20 | 9.7 |
| 4 | Phenol | n-hexyl | n-hexyl | 2.7 | — | 20 | 7.7 |
| 5 | m-Cresol | — | — | — | — | 20 | 8.9 |
| 6 | m-Cresol | n-Bu | n-Bu | 2.7 | — | 20 | 12.1 |
| 7 | m-Cresol | n-Bu | n-Bu | 2.7 | 3.8 | 20 | 17.3 |
| 8 | m-Cresol | n-Bu | iso-Bu | 2.7 | 3.8 | 20 | 17.2 |
| 9 | m-Cresol | n-Bu | sec-Bu | 2.7 | 3.8 | 20 | 17.1 |
| 10 | m-Cresol | n-Bu | tert-Bu | 2.7 | 3.8 | 20 | 8.7 |
| Entry | Catalyst | Spacer Length (n) | 3:2 Ratio Produced in Presence of the Catalyst | |||
|---|---|---|---|---|---|---|
| Phenol | o-Cresol | m-Cresol b | m-Xylenol | |||
| 1 | 17 | 6 | 12.3 | 20.6 | 15.5 (13.0) c | 11.7 |
| 2 | 20 | 3 | 9.6 | 16.6 | 13.0 c | 19.1 |
| 3 | 21 | 3 | 8.7 | 18.7 | 10.1 | 23.8 |
| 4 | 22 | 6 | 10.2 | 14.0 | 10.1 | 14.9 |
| Entry | Polymer Number | m of Dibromide 24 | n of Dithiol 23 |
|---|---|---|---|
| 1 | 25a | 2 | 2 |
| 2 | 25b | 3 | 3 |
| 3 | 25c | 4 | 4 |
| 4 | 25d | 6 | 6 |
| 5 | 25e | 8 | 8 |
| 6 | 25f | 3 | 6 |
| 7 | 25g | 3 | 9 |
| 8 | 25h | 3 | 12 |
| 9 | 25i | 4 | 12 |
| 10 | 25j | 6 | 12 |
| 11 | 25k | 8 | 12 |
| Entry | Polymer Number | m of Dibromide 24 | n of Dibromide 28 | Average Number of Alkylene Sulphide Units in the Polymer (p) |
|---|---|---|---|---|
| 1 | 26a | 2 | — | Not known b |
| 2 | 26b | 3 | — | 24 |
| 3 | 26c | 4 | — | 43 |
| 4 | 26d | 6 | — | 33 |
| 5 | 26e | 8 | — | 20 c |
| 6 | 27a | 3 | 6 | Not known |
| 7 | 27b | 6 | 8 | Not known |
| Entry | Substrate | Catalyst (mg) | Lewis Acid (mg) | SO2Cl2 (mmol) | Components of Product Mixture (Absolute %) | |||
|---|---|---|---|---|---|---|---|---|
| 2-Cl | 4-Cl | Others c | para/ortho Ratio | |||||
| 1 | o-Cresol | 26b (3) | AlCl3 (20) | 105 | 4.5 | 95.1 | 0.1 | 21.4 |
| 2 | m-Cresol | 27a (3) | AlCl3 (50) | 105 | 7.7 | 91.6 | 0.3 | 11.9 |
| 3 | m-Xylenol b | 26d (3) | FeCl3 (20) | 105 | 5.6 | 93.5 | 0.8 | 16.7 |
| 4 | m-Xylenol b | 26d (5) | FeCl3 (50) | 105 | 2.8 | 94.9 | 2.2 | 33.9 |
| 5 | Phenol | 27a (20) | AlCl3 (50) | 110 | 7.5 | 90.8 | 1.5 | 12.0 |
| 6 | Phenol (di) | 26a (20) | AlCl3 (30) | 210 | 0.8 | 91.8 | 7.0 | 109.3 |
| 7 | Phenol (di) | 26a (50) | AlCl3 (100) | 210 | 0.7 | 94.0 | 5.0 | 134.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, K.; El-Hiti, G.A. Development of Efficient and Selective Processes for the Synthesis of Commercially Important Chlorinated Phenols. Organics 2021, 2, 142-160. https://0-doi-org.brum.beds.ac.uk/10.3390/org2030012
Smith K, El-Hiti GA. Development of Efficient and Selective Processes for the Synthesis of Commercially Important Chlorinated Phenols. Organics. 2021; 2(3):142-160. https://0-doi-org.brum.beds.ac.uk/10.3390/org2030012
Chicago/Turabian StyleSmith, Keith, and Gamal A. El-Hiti. 2021. "Development of Efficient and Selective Processes for the Synthesis of Commercially Important Chlorinated Phenols" Organics 2, no. 3: 142-160. https://0-doi-org.brum.beds.ac.uk/10.3390/org2030012