Synthesis of 1-Trifluorometylindanes and Close Structures: A Mini Review
Abstract
:1. Introduction
2. Discussion
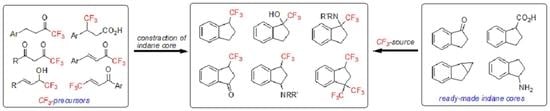
2.1. Construction of Trifluoromethylindane Core from CF3 Precursors
2.2. Trifluoromethylation of Indane Scaffolds
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hiyama, T. Organofluorine Compounds. Chemistry and Applications; Springer: Berlin, Germany, 2000. [Google Scholar]
- Chambers, R.D. Fluorine in Organic Chemistry; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Uneyama, K. Organofluorine Chemistry; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Theodoridis, G. Fluorine-Containing Agrochemicals: An Overview of Recent Developments. In Advances in Fluorine Science; Tressaud, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 121–175. [Google Scholar]
- Begue, J.P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tressaud, A.; Haufe, G. (Eds.) Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Petrov, V.A. (Ed.) Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nenajdenko, V.G. (Ed.) Fluorine in Heterocyclic Chemistry; Springer: Berlin, Germany, 2014. [Google Scholar]
- Prakash, R.V. Organofluorine Compounds in Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Westwell, A.D. (Ed.) Fluorinated Pharmaceuticals: Advances in Medicinal Chemistry; Future Science Ltd.: London, UK, 2015. [Google Scholar]
- Groult, H.; Leroux, F.R.; Tressaud, A. (Eds.) Modern Synthesis Processes and Reactivity of Fluorinated Compounds. Progress in Fluorine Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Postigo, A. (Ed.) Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Prasher, P.; Sharma, M. Medicinal chemistry of indane and its analogues: A mini review. ChemistrySelect 2021, 6, 2658. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L. Recent advances in the synthesis of indanes and indenes. Chem. Eur. J. 2016, 22, 5056. [Google Scholar] [CrossRef] [PubMed]
- Borie, C.; Ackermann, L.; Nechab, M. Enantioselective syntheses of indanes: From organocatalysis to C-H functionalization. Chem. Soc. Rev. 2016, 45, 1368. [Google Scholar] [CrossRef]
- Kuck, D. Three-dimensional hydrocarbon cores based on multiply fused cyclopentane and indane units: Centropolyindanes. Chem. Rev. 2006, 106, 4885. [Google Scholar] [CrossRef] [PubMed]
- Ganellin, C.R. Indane and indene derivatives of biological interest. Adv. Drug Res. 1967, 4, 163. [Google Scholar]
- Iakovenko, R.O.; Kazakova, A.N.; Muzalevskiy, V.M.; Ivanov, A.Y.; Boyarkaya, I.A.; Chicca, A.; Petrucci, V.; Gertsch, J.; Krasavin, M.; Starova, G.L.; et al. Reactions of CF3-enones with arenes under super-lectrophilic activation: A pathway to trans-1,3-diaryl-1-CF3-indanes, new cannabinoid receptor ligands. Org. Biomol. Chem. 2015, 13, 8827. [Google Scholar] [CrossRef]
- Iakovenko, R.O.; Chicca, A.; Nieri, D.; Reynoso-Moreno, I.; Gertsch, J.; Krasavin, M.Y.; Vasilyev, A.V. Synthesis of various arylatedtrifluoromethyl substituted indanes and indenes, and study of their biological activity. Tetrahedron 2019, 75, 624. [Google Scholar] [CrossRef]
- Aubert, C.; Begue, J.-P.; Bonnet-Delpon, D.; Mesureur, D. Access to trifluoromethylindanes; cycloalkylation of β-phenyl trifluoromethyl ketones, β-keto esters, and alcohols. J. Chem. Soc. Perkin Trans. 1 1989, 3, 395. [Google Scholar] [CrossRef]
- Abouadbellah, A.; Aubert, C.; Begue, J.-P.; Bonnet-Delpon, D.; Guilhem, J. Lewis acid-induced ene-cyclization of ω-olefinictrifluoromethyl ketones: Access to alicyclic compounds bearing a CF3 group. J. Chem. Soc. Perkin Trans. 1 1991, 6, 1397. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Paknia, F.; Narayanan, A.; Rasul, G.; Mathew, T.; Olah, G.A. Efficient synthesis of trifluoromethylateddihydrochalcones, aryl vinyl ketones and indanones by superelectrophilic activation of 4,4,4-trifluoro/3-(trifluoromethyl)crotonic acids. J. Fluor. Chem. 2012, 143, 292. [Google Scholar] [CrossRef]
- Dong, K.; Li, Y.; Wang, Z.; Ding, K. Catalytic asymmetric hydrogenation of α-CF3- or β-CF3-substituted acrylic acids using rhodium(I) complexes with a combination of chiral and achiral ligands. Angew. Chem. Int. Ed. 2013, 52, 14191. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Boblak, K.N.; Topinka, M.J.; Kindelin, P.J.; Briski, J.M.; Zheng, C.; Klumpp, D.A. Superelectrophiles and the effects of trifluoromethyl substituents. J. Am. Chem. Soc. 2010, 132, 3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakovenko, R.O.; Muzalevskiy, V.M.; Nenajdenko, V.G.; Vasilyev, A.V. Reactions of 1,1,1-trifluoro-4-phenylbut-3-en-2-one with arenestrifluoromethanesulfonic acid. Russ. J. Org. Chem. 2015, 51, 436. [Google Scholar] [CrossRef]
- Iakovenko, R.O.; Kazakova, A.N.; Boyarkaya, I.A.; Gurzhiy, V.V.; Adontceva, M.S.; Panikorovskiy, T.L.; Muzalevskiy, V.M.; Nenajdenko, V.G.; Vasilyev, A.V. Superacid-promoted synthesis of CF3-indenes using brominated CF3-enones. Eur. J. Org. Chem. 2017, 37, 5632. [Google Scholar] [CrossRef]
- Kazakova, A.N.; Iakovenko, R.O.; Boyarskaya, I.A.; Nenajdenko, V.G.; Vasilyev, A.V. Acid-promoted reaction of trifluoromethylatedallyl alcohols with arenes. Stereoselective synthesis of CF3-alkenes and CF3-indanes. J. Org. Chem. 2015, 80, 9506. [Google Scholar] [CrossRef] [Green Version]
- Zerov, A.V.; Bulova, A.A.; Khoroshilova, O.V.; Vasilyev, A.V. TfOH-promoted transformations of TMS-ethers of diarylsubstituted CF3-allyl alcohols with arenes into CF3-indanes. Org. Chem. Front. 2019, 6, 3264. [Google Scholar] [CrossRef]
- Kazakova, A.N.; Iakovenko, R.O.; Boyarskaya, I.A.; Ivanov, A.Y.; Avdontceva, M.S.; Zolotarev, A.A.; Panikorovsky, T.L.; Starova, G.L.; Nenajdenko, V.G.; Vasilyev, A.V. Brominated CF3-allyl alcohols as multicentered electrophiles in TfOH promoted reactions with arenes. Org. Chem. Front. 2017, 4, 255. [Google Scholar] [CrossRef]
- Sinyakov, V.R.; Mezhenkova, T.V.; Karpov, V.M.; Platonov, V.E. The alicyclic ring contraction of perfluoro-1-phenyltetralin in reaction with antimony pentafluoride. J. Fluor. Chem. 2004, 125, 49. [Google Scholar] [CrossRef]
- Mezhenkova, T.V.; Karpov, V.M.; Zonov, Y.V. Formation of polyfluorofluorenes in the reactions of perfluoro-1,1-diphenylalkanes with antimony pentafluoride. J. Fluor. Chem. 2018, 207, 59. [Google Scholar] [CrossRef]
- Liu, B.; Hu, P.; Zhang, Y.; Li, Y.; Bai, D.; Li, X. Rh(III)-catalyzed diastereodivergentspiroannulation of cyclic imines with activated alkenes. Org. Lett. 2017, 19, 5402. [Google Scholar] [CrossRef]
- Chaudhary, B.; Autim, P.; Shinde, S.D.; Yakkala, P.A.; Giri, D.; Sharma, S. Rh(III)-catalyzed [3+2] annulations via C–H activation: Direct access to trifluoromethyl substituted indenamines and aminoindanes. Org. Lett. 2019, 21, 2763. [Google Scholar] [CrossRef]
- Hu, T.; Xu, Y.; Zhang, S.; Xiong, H.-Y.; Zhang, G. Synthesis of β-amino esters with an undane backbone by rhenium-catalyzed [3+2] annulations. Org. Lett. 2020, 22, 8666. [Google Scholar] [CrossRef]
- Kundu, K.; McCullagh, J.V.; Morehead, A.T. Hydroacylation of 2-vinyl benzaldehyde system: An afficient method for the synthesis of chiral 3-substituted indanones. J. Am. Chem. Soc. 2005, 127, 16042. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, H.; Muramatsu, H.; Inukai, K. Synthesis of fluorine-containing indanes and indenes: A novel radical cycloaddition of alkylbenzenes and hexafluoropropene. Chem. Lett. 1974, 3, 791. [Google Scholar] [CrossRef]
- Kimoto, H.; Muramatsu, H.; Inukai, K. Synthesis and reactions of fluorine-containing aromatic ketones. Bull. Chem. Soc. Jpn. 1976, 49, 1642. [Google Scholar] [CrossRef] [Green Version]
- Haszeldine, R.N.; Mitchinson, A.J.; Rowland, R.; Tipping, A.E. Fluoro-olefin chemistry. Part IX. Thermal insertion of hexafluoropropene into carbon-hydrogen bonds in alkylbenzenes. J. Chem. Soc. Perkin Trans. 1 1976, 5, 517. [Google Scholar] [CrossRef]
- Chuikov, I.P.; Karpov, V.M.; Platonov, V.E.; Yakobson, G.G. Cyclization of difluorocarbene to perfluoro-3-methylindene and perfluoro-1-methylindane. Thermolysis and bromination of the resultant cyclopropane derivatives. Russ. Chem. Bull. 1988, 37, 1645. [Google Scholar] [CrossRef]
- Furukawa, T.; Nishimine, T.; Tokunaga, E.; Hasegawa, K.; Shiro, M.; Shibata, N. Organocatalyzed region- and enantioselectiveallylictrifluoromethylation of Morita-Baylis-Hilman adducts using Ruppert-Prakash reagent. Org. Lett. 2011, 13, 3972. [Google Scholar] [CrossRef]
- Rabasa-Alcaniz, F.; Asensio, A.; Sanchez-Rosello, M.; Escolano, M.; del Poza, C.; Fustero, S. Intramolecular nitronecycloaddition of α-(trifluoromethyl)styrenes. Role of the CF3 group in the regioselectivity. J. Org. Chem. 2017, 82, 2505. [Google Scholar] [CrossRef]
- Bardin, V.V.; Petrov, V.A.; Stennikova, I.V.; Furin, G.G.; German, L.S. Formation of perfluoro-1,1,3-trimethylindane in the reaction of trimethylsilylpentafluorobenzene with perfluoro-4-methyl-2-pentene. Russ. Chem. Bull. 1989, 38, 420. [Google Scholar] [CrossRef]
- Gassman, P.G.; Ray, J.A.; Wenthold, P.G.; Mickelson, J.W. Synthesis of perfluoroalkeylatedindenes. J. Org. Chem. 1991, 56, 5143. [Google Scholar] [CrossRef]
- Muzita, S.; Shibata, N.; Akiti, S.; Fujimoto, H.; Nakamura, S.; Toru, T. Cinchona alkaloids/TMAF combination-catalyzed nucleophilic enentioselectivetrifluoromethylation of aryl ketones. Org. Lett. 2007, 9, 3707. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, R.J.; Brewster, J.H.; Smith, H.E. Application of the bezene sector and the benzene chirality rulus to perhydrobenzocycloalkenes and related compounds. J. Am. Chem. Soc. 1992, 114, 2181. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, H.; Jiang, F.; Fang, Y.; Zhu, L.; Li, C. Copper-catalyzed ring-opening 1,3-amonotrifluoromethylation of arylcyclopropanes. Org. Lett. 2021, 23, 2268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, H.; Xiao, H.; Wang, Z.; Zhu, L.; Li, C. Copper-catalyzed ring-opening radical trifluoromethylation of cycloalkanoneoximes. Org. Lett. 2019, 21, 5201. [Google Scholar] [CrossRef] [PubMed]
- Sarver, P.J.; Bacauanu, V.; Schultz, D.M.; DiRocco, D.A.; Lam, Y.; Sherer, E.C.; NacMillan, D.W.C. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)–H trifluoromethylation. Nat. Chem. 2020, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.-D.; Cui, C.-C.; Gui, C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Metal-catalyzed spiroannulation-fluoromethylfunctionalization of, 1,5-enynes for the synthesis of stereodefined (Z)-epiroindenes. Chem. Asian J. 2020, 15, 4070. [Google Scholar] [CrossRef]
- Leonard, N.G.; Palmer, W.N.; Friedfield, M.R.; Bezdek, M.J.; Chirik, P.J. Remote, diastereoselective cobalt-catalyzed alkene isomerization-hydroboration: Access to stereodefined 1,3-difunctionalyzed indanes. ACS Catal. 2019, 9, 9034. [Google Scholar] [CrossRef]




































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoroshilova, O.V.; Vasilyev, A.V. Synthesis of 1-Trifluorometylindanes and Close Structures: A Mini Review. Organics 2021, 2, 348-364. https://0-doi-org.brum.beds.ac.uk/10.3390/org2040019
Khoroshilova OV, Vasilyev AV. Synthesis of 1-Trifluorometylindanes and Close Structures: A Mini Review. Organics. 2021; 2(4):348-364. https://0-doi-org.brum.beds.ac.uk/10.3390/org2040019
Chicago/Turabian StyleKhoroshilova, Olesya V., and Aleksander V. Vasilyev. 2021. "Synthesis of 1-Trifluorometylindanes and Close Structures: A Mini Review" Organics 2, no. 4: 348-364. https://0-doi-org.brum.beds.ac.uk/10.3390/org2040019