The Role of Cathepsins in the Growth of Primary and Secondary Neoplasia in the Bone
Abstract
:1. Introduction
1.1. Osteosarcoma
1.2. Bone Metastasis in Breast and Prostate Cancer
2. Proteolytic Enzyme Targeting in Cancer
2.1. The Physiologic Role of Cathepsins
2.2. Role of Cathepsins in Bone Remodeling
3. The Role of Cathepsin in Cancer
The Role of Cathepsins in Primary Bone Cancer and Metastases to the Bone
4. Targeting Cathepsin in Primary Bone and Metastatic Bone Cancer
4.1. Cathepsin Targeting in Primary Bone Tumors
4.2. Cathepsin Targeting in Secondary Bone Tumors
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCa | Breast Cancer |
| BMP-4 | Bone Morphogenic Protein 4 |
| CAM | Cell Adhesion Molecule |
| Cat | Cathepsin |
| CCL2 | Macrophage Chemo Attractant Protein-1 |
| CCR2 | C-C Chemokine Receptor Type 2 |
| CSF | Colony Stimulating Factor |
| Dox | Doxorubicin |
| ECM | Extracellular Matrix |
| GPCR | G Protein Coupled Receptor |
| hOSM | Oncostatin M |
| IGF | Insulin-Like Growth Factor |
| IL | Interleukin |
| m-CSF | Macrophage Colony Stimulating Factor |
| MDSC | Myeloid-Derived Suppressor Cells |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| OS | Osteosarcoma |
| PAR-1 | Protease-Activated Receptor-1 |
| PCa | Prostate Cancer |
| PTH | Parathyroid Hormone |
| PTHrP | Parathyroid Hormone Related Peptide |
| RANKL | Receptor Activator of NF-Kappa-β Ligand |
| SCID | Severe Combined Immunodeficiency |
| sRANKL | Soluble Receptor Activator of NF-Kappa-β Ligand |
| TGF-β | Transforming Growth Factor-beta |
| TNFα | Tumor Necrosis Factor-alpha |
| TRAIL-DR5 | Tumor Necrosis Factor-Related Apoptosis- Inducing Ligand Death Receptor-5 |
| TRACP 5b | Tartrate-Resistant Acid Phosphatase 5b |
| uPA | Urokinase-Plasminogen Activator |
| VEGF | Vascular Endothelial Growth Factor |
| ZOL | Zoledronic Acid |
References
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Ries, L.A.G. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; Smith, M.A., Gurney, J.G., Linet, M., Tamra, T., Young, J.L., Bunin, G.R., Eds.; National Cancer Institute (NCI): Bethesda, MA, USA, 1999. [Google Scholar]
- Linabery, A.M.; Ross, J.A. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008, 113, 2575–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar] [PubMed]
- Theriault, R.L. Biology of bone metastases. Cancer Control 2012, 19, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, W.S.; Goorin, A.M. Current treatment of osteosarcoma. Cancer Investig. 2001, 19, 292–315. [Google Scholar] [CrossRef]
- Fuchs, B.; Pritchard, D.J. Etiology of osteosarcoma. Clin. Orthop Relat Res. 2002, 397, 40–52. [Google Scholar] [CrossRef]
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004, 9, 422–441. [Google Scholar] [CrossRef]
- Smith, M.A.; Seibel, N.L.; Altekruse, S.F.; Ries, L.A.; Melbert, D.L.; O’Leary, M.; Smith, F.O.; Reaman, G.H. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J. Clin. Oncol. 2010, 28, 2625–2634. [Google Scholar] [CrossRef]
- Ferrari, S.; Serra, M. An update on chemotherapy for osteosarcoma. Expert Opin. Pharmacother. 2015, 16, 2727–2736. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Awolaran, O.; Brooks, S.A.; Lavender, V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 2016, 30, 156–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucci, N.; Teti, A. Osteomimicry: How tumor cells try to deceive the bone. Front. Biosci. 2010, 2, 907–915. [Google Scholar]
- Barnes, G.L.; Hebert, K.E.; Kamal, M.; Javed, A.; Einhorn, T.A.; Lian, J.B.; Stein, G.S.; Gerstenfeld, L.C. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res. 2004, 64, 4506–4513. [Google Scholar] [CrossRef] [Green Version]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Kakhki, V.R.; Anvari, K.; Sadeghi, R.; Mahmoudian, A.S.; Torabian-Kakhki, M. Pattern and distribution of bone metastases in common malignant tumors. Nucl. Med. Rev. Cent. East Eur. 2013, 16, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Dhillon, S.; Lyseng-Williamson, K.A. Zoledronic acid: A review of its use in the management of bone metastases of malignancy. Drugs 2008, 68, 507–534. [Google Scholar] [CrossRef]
- Rubens, R.D. Metastatic breast cancer. Curr. Opin. Oncol. 1995, 7, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Jensen, A.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sørensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. Discov. Med. 2004, 4, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.J.; Singh, R.K. Proteases as modulators of tumor-stromal interaction: Primary tumors to bone metastases. Biochim. Biophys. Acta 2008, 1785, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Onishi, T.; Hayashi, N.; Theriault, R.L.; Hortobagyi, G.N.; Ueno, N.T. Future directions of bone-targeted therapy for metastatic breast cancer. Nat. Rev. Clin. Oncol. 2010, 7, 641–651. [Google Scholar] [CrossRef]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Saylor, P.J.; Smith, M.R. Treatment and prevention of bone complications from prostate cancer. Bone 2011, 48, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Roato, I.; D’Amelio, P.; Gorassini, E.; Grimaldi, A.; Bonello, L.; Fiori, C.; Delsedime, L.; Tizzani, A.; De Libero, A.; Isaia, G.; et al. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS ONE. 2008, 3, e3627. [Google Scholar] [CrossRef]
- Palermo, C.; Joyce, J.A. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol. Sci. 2008, 29, 22–28. [Google Scholar] [CrossRef]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. Matrix metalloproteinases as valid clinical targets. Curr. Pharm. Des. 2007, 13, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Overall, C.M.; Kleifeld, O. Tumour microenvironment—opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef]
- Overall, C.M.; López-Otín, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Zucker, S.; Cao, J.; Chen, W.T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 2000, 19, 6642–6650. [Google Scholar] [CrossRef]
- Krüger, A.; Kates, R.E.; Edwards, D.R. Avoiding spam in the proteolytic internet: Future strategies for anti-metastatic MMP inhibition. Biochim. Biophys. Acta 2010, 1803, 95–102. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; A Hoeben, K.; Jansen, I.D.; Brömme, D.; Cleutjens, K.B.; Heeneman, S.; Peters, C.; Reinheckel, T.; Saftig, P.; et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: Differences between calvaria and long bone. J. Bone Miner. Res. 2006, 21, 1399–1408. [Google Scholar] [CrossRef]
- Abboud-Jarrous, G.; Atzmon, R.; Peretz, T.; Palermo, C.; Gadea, B.B.; Joyce, J.A.; Vlodavsky, I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J. Biol. Chem. 2008, 283, 18167–18176. [Google Scholar] [CrossRef] [Green Version]
- Goretzki, L.; Schmitt, M.; Mann, K.; Calvete, J.J.; Chucholowski, N.; Kramer, M.; Günzler, W.A.; Jänicke, F.; Graeff, H. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Lett. 1992, 297, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Aisa, M.C.; Rahman, S.; Senin, U.; Maggio, D.; Russell, R.G.G. Cathepsin B activity in normal human osteoblast-like cells and human osteoblastic osteosarcoma cells (MG-63): Regulation by interleukin-1-beta and parathyroid hormone. Biochim. Biophys. Acta 1996, 1290, 29–36. [Google Scholar] [CrossRef]
- Repnik, U.; Stoka, V.; Turk, V.; Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 2012, 1824, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Krueger, S.; Haeckel, C.; Buehling, F.; Roessner, A. Inhibitory effects of antisense cathepsin B cDNA transfection on invasion and motility in a human osteosarcoma cell line. Cancer Res. 1999, 59, 6010–6014. [Google Scholar]
- Sloane, B.F.; Rozhin, J.; Johnson, K.; Taylor, H.; Crissman, J.D.; Honn, K.V. Cathepsin B: Association with plasma membrane in metastatic tumors. Proc. Natl. Acad. Sci. USA 1986, 83, 2483–2487. [Google Scholar] [CrossRef] [Green Version]
- Haeckel, C.; Ayala, A.G.; Radig, K.; Raymond, A.K.; Roessner, A.; Czerniak, B. Protease expression in dedifferentiated parosteal osteosarcoma. Arch. Pathol. Lab. Med. 1999, 123, 213–221. [Google Scholar]
- Köppel, P.; Baici, A.; Keist, R.; Matzku, S.; Keller, R. Cathepsin B-like proteinase as a marker for metastatic tumor cell variants. Exp. Cell Biol. 1984, 52, 293–299. [Google Scholar]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 42, D503–D509. [Google Scholar] [CrossRef] [Green Version]
- Rochefort, H.; Capony, F.; Garcia, M. Cathepsin D: A protease involved in breast cancer metastasis. Cancer Metastasis Rev. 1990, 9, 321–331. [Google Scholar] [CrossRef]
- Conover, C.A.; Perry, J.E.; Tindall, D.J. Endogenous cathepsin D-mediated hydrolysis of insulin-like growth factor-binding proteins in cultured human prostatic carcinoma cells. J. Clin. Endocrinol. Metab. 1995, 80, 987–993. [Google Scholar]
- Van der Stappen, J.W.; Williams, A.C.; Maciewicz, R.A.; Paraskeva, C. Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D. Int. J. Cancer 1996, 67, 547–554. [Google Scholar] [CrossRef]
- Wilson, T.J.; Nannuru, K.C.; Singh, R.K. Cathepsin G recruits osteoclast precursors via proteolytic activation of protease-activated receptor-1. Cancer Res. 2009, 69, 3188–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafatian, N.; Karunakaran, D.; Rayner, K.J.; Leenen, F.H.; Milne, R.W.; Whitman, S.C. Cathepsin G deficiency decreases complexity of atherosclerotic lesions in apolipoprotein E-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1141–H1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husmann, K.; Muff, R.; Bolander, M.E.; Sarkar, G.; Born, W.; Fuchs, B. Cathepsins and osteosarcoma: Expression analysis identifies cathepsin K as an indicator of metastasis. Mol. Carcinog. 2008, 47, 66–73. [Google Scholar] [CrossRef]
- Rojnik, M.; Jevnikar, Z.; Mirković, B.; Janeš, D.; Zidar, N.; Kikelj, D.; Kos, J. Cathepsin H indirectly regulates morphogenetic protein-4 (BMP-4) in various human cell lines. Radiol. Oncol. 2011, 45, 259–266. [Google Scholar] [CrossRef]
- Ketterer, S.; Gomez-Auli, A.; Hillebrand, L.E.; Petrera, A.; Ketscher, A.; Reinheckel, T. Inherited diseases caused by mutations in cathepsin protease genes. FEBS J. 2017, 284, 1437–1454. [Google Scholar] [CrossRef] [Green Version]
- Littlewood-Evans, A.J.; Bilbe, G.; Bowler, W.; Farley, D.; Wlodarski, B.; Kokubo, T.; Inaoka, T.; Sloane, J.; Evans, D.B.; A Gallagher, J. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. 1997, 57, 5386–5390. [Google Scholar]
- Vääräniemi, J.; Halleen, J.M.; Kaarlonen, K.; Ylipahkala, H.; Alatalo, S.L.; Andersson, G.; Kaija, H.; Vihko, P.; Väänänen, H.K. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J. Bone Miner. Res. 2004, 19, 1432–1440. [Google Scholar] [CrossRef]
- Tezuka, K.; Tezuka, Y.; Maejima, A.; Sato, T.; Nemoto, K.; Kamioka, H.; Hakeda, Y.; Kumegawa, M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J. Biol. Chem. 1994, 269, 1106–1109. [Google Scholar]
- Inaoka, T.; Bilbe, G.; Ishibashi, O.; Tezuka, K.; Kumegawa, M.; Kokubo, T. Molecular cloning of human cDNA for cathepsin K: Novel cysteine proteinase predominantly expressed in bone. Biochem. Biophys. Res. Commun. 1995, 206, 89–96. [Google Scholar] [CrossRef]
- Li, Y.P.; Alexander, M.; Wucherpfennig, A.L.; Yelick, P.; Chen, W.; Stashenko, P. Cloning and complete coding sequence of a novel human cathepsin expressed in giant cells of osteoclastomas. J. Bone Miner. Res. 1995, 10, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.J.; Tomaszek, T.A.; Thompson, S.K.; Amegadzie, B.Y.; Hanning, C.R.; Jones, C.; Kurdyla, J.T.; McNulty, D.E.; Drake, F.H.; Gowen, M.; et al. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J. Biol. Chem. 1996, 271, 12517–12524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelb, B.D.; Shi, G.P.; Chapman, H.A.; Desnick, R.J. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 1996, 273, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; Von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [Green Version]
- Duong, L.T. Therapeutic inhibition of cathepsin K-reducing bone resorption while maintaining bone formation. Bonekey Rep. 2012, 1, 67. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.G.; Cusano, N.E.; Silva, B.C.; Cremers, S.; Bilezikian, J.P. Cathepsin K: Its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 2011, 7, 447–456. [Google Scholar] [CrossRef]
- Everts, V.; Aronson, D.C.; Beertsen, W. Phagocytosis of bone collagen by osteoclasts in two cases of pycnodysostosis. Calcif. Tissue Int. 1985, 37, 25–31. [Google Scholar] [CrossRef]
- Everts, V.; Hou, W.S.; Rialland, X.; Tigchelaar, W.; Saftig, P.; Gelb, B.D.; Beertsen, W. Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif. Tissue Int. 2003, 73, 380–386. [Google Scholar] [CrossRef]
- Turan, S. Current research on pycnodysostosis. Intractable Rare Dis Res. 2014, 3, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.J.; Kirschke, H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981, 80, 535–561. [Google Scholar] [PubMed]
- Hsing, L.C.; Rudensky, A.Y. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol. Rev. 2005, 207, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zavasnik-Bergant, T.; Turk, B. Cysteine cathepsins in the immune response. Tissue Antigens 2006, 67, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, Y.; Kaleta, J.; Brömme, D. The role of cathepsins in osteoporosis and arthritis: Rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 973–993. [Google Scholar] [CrossRef]
- Salminen-Mankonen, H.J.; Morko, J.; Vuorio, E. Role of cathepsin K in normal joints and in the development of arthritis. Curr. Drug Targets 2007, 8, 315–323. [Google Scholar] [CrossRef]
- Georges, S.; Ruiz Velasco, C.; Trichet, V.; Fortun, Y.; Heymann, D.; Padrines, M. Proteases and bone remodelling. Cytokine Growth Factor Rev. 2009, 20, 29–41. [Google Scholar] [CrossRef]
- Garnero, P.; Ferreras, M.; Karsdal, M.; NicAmhlaoibh, R.; Risteli, J.; Borel, O.; Qvist, P.; Delmas, P.; Foged, N.; Delaissé, J.-M. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003, 18, 859–867. [Google Scholar] [CrossRef]
- Mandelin, J.; Hukkanen, M.; Li, T.-F.; Korhonen, M.; Liljeström, M.; Sillat, T.; Hanemaaijer, R.; Salo, J.; Santavirta, S.; Konttinen, Y.T. Human osteoblasts produce cathepsin K. Bone 2006, 38, 769–777. [Google Scholar] [CrossRef]
- Kimura, S.; Sato, Y.; Matsubara, H.; Adachi, I.; Yamaguchi, K.; Suzuki, M.; Suemasu, K.; Abe, K. A retrospective evaluation of the medical treatment of malignancy-associated hypercalcemia. Jpn. J. Cancer Res. 1986, 77, 85–91. [Google Scholar]
- Rubens, R.D. Bone metastases—The clinical problem. Eur. J. Cancer 1998, 34, 210–213. [Google Scholar] [CrossRef]
- Delmas, P.D.; Fontana, A. Bone loss induced by cancer treatment and its management. Eur. J. Cancer 1998, 34, 260–262. [Google Scholar] [CrossRef]
- Kakegawa, H.; Nikawa, T.; Tagami, K.; Kamioka, H.; Sumitani, K.; Kawata, T.; Drobnič-Kosorok, M.; Lenarčič, B.; Turk, V.; Katunuma, N. Participation of cathepsin L on bone resorption. FEBS Lett. 1993, 321, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Leto, G.; Crescimanno, M.; Flandina, C.; Sepporta, M.V.; Tumminello, F.M. Cathepsin L in Normal and Pathological Bone Remodeling. Clin. Rev. Bone Miner. Metab. 2011, 9, 107–121. [Google Scholar] [CrossRef]
- Nakase, T.; Kaneko, M.; Tomita, T.; Myoui, A.; Ariga, K.; Sugamoto, K.; Uchiyama, Y.; Ochi, T.; Yoshikawa, H. Immunohistochemical detection of cathepsin D, K, and L in the process of endochondral ossification in the human. Histochem. Cell Biol. 2000, 114, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Brage, M.; Abrahamson, M.; Lindström, V.; Grubb, A.; Lerner, U.H. Different cysteine proteinases involved in bone resorption and osteoclast formation. Calcif. Tissue Int. 2005, 76, 439–447. [Google Scholar] [CrossRef]
- Goto, T.; Tsukuba, T.; Kiyoshima, T.; Nishimura, Y.; Kato, K.; Yamamoto, K.; Tanaka, T. Immunohistochemical localization of cathepsins B, D and L in the rat osteoclast. Histochemistry 1993, 99, 411–414. [Google Scholar] [CrossRef]
- Evans, P.; Etherington, D.J. Characterisation of cathepsin B and collagenolytic cathepsin from human placenta. Eur. J. Biochem. 1978, 83, 87–97. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Barrett, A.J.; Lazarus, G.S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem. J. 1974, 137, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Buck, M.R.; Karustis, D.G.; Day, N.A.; Honn, K.V.; Sloane, B.F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 1992, 282, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.; Ward, R.; Gavrilovic, J.; Atkinson, S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992, 1, 224–230. [Google Scholar] [PubMed]
- Kobayashi, H.; Schmitt, M.; Goretzki, L.; Chucholowski, N.; Calvete, J.; Kramer, M.; Günzler, W.A.; Jänicke, F.; Graeff, H. Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA). J. Biol. Chem. 1991, 266, 5147–5152. [Google Scholar] [PubMed]
- Jiao, W.J.; Xu, J.; Pan, H.; Wang, T.Y.; Shen, Y. Effect of endothelin-1 in esophageal squamous cell carcinoma invasion and its correlation with cathepsin B. World J. Gastroenterol. 2007, 13, 4002–4005. [Google Scholar] [CrossRef] [Green Version]
- Edgington-Mitchell, L.E.; Rautela, J.; Duivenvoorden, H.M.; Jayatilleke, K.M.; Van Der Linden, W.A.; Verdoes, M.; Bogyo, M.; Parker, B.S. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget 2015, 6, 27008–27022. [Google Scholar] [CrossRef] [PubMed]
- Herroon, M.K.; Rajagurubandara, E.; Rudy, D.L.; Chalasani, A.; Hardaway, A.L.; Podgorski, I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene 2013, 32, 1580–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiviranta, R.; Morko, J.; Alatalo, S.L.; NicAmhlaoibh, R.; Risteli, J.; Laitala-Leinonen, T.; Vuorio, E. Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone 2005, 36, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Peters, C.; Saftig, P.; Brömme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [Green Version]
- Sudhan, D.R.; Pampo, C.; Rice, L.; Siemann, D.W. Cathepsin L inactivation leads to multimodal inhibition of prostate cancer cell dissemination in a preclinical bone metastasis model. Int. J. Cancer 2016, 138, 2665–2677. [Google Scholar] [CrossRef] [Green Version]
- Brage, M.; Lie, A.; Ransjö, M.; Kasprzykowski, F.; Kasprzykowska, R.; Abrahamson, M.; Grubb, A.; Lerner, U. Osteoclastogenesis is decreased by cysteine proteinase inhibitors. Bone 2004, 34, 412–424. [Google Scholar] [CrossRef]
- Patel, N.; Nizami, S.; Song, L.; Mikami, M.; Hsu, A.; Hickernell, T.; Chandhanayingyong, C.; Rho, S.; Compton, J.; Caldwell, J.-M.; et al. CA-074Me compound inhibits osteoclastogenesis via suppression of the NFATc1 and c-FOS signaling pathways. J. Orthop. Res. 2015, 33, 1474–1486. [Google Scholar] [CrossRef]
- Strålberg, F.; Henning, P.; Gjertsson, I.; Kindlund, B.; Souza, P.P.C.; Persson, E.; Abrahamson, M.; Kasprzykowski, F.; Grubb, A.; Lerner, U. Cysteine proteinase inhibitors regulate human and mouse osteoclastogenesis by interfering with RANK signaling. FASEB J. 2013, 27, 2687–2701. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Trejo, J. Protease-activated receptor signaling: New roles and regulatory mechanisms. Curr. Opin. Hematol. 2007, 14, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kamiyama, M.; Tani-Ishii, N.; Suzuki, H.; Ichikawa, Y.; Hamaguchi, Y.; Momiyama, N.; Shimada, H. Inhibition of osteoclast differentiation and bone resorption by cathepsin K antisense oligonucleotides. Mol. Carcinog. 2001, 32, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Grabowskal, U.; Chambers, T.J.; Shiroo, M. Recent developments in cathepsin K inhibitor design. Curr. Opin. Drug Discov. Dev. 2005, 8, 619–630. [Google Scholar]
- Stoch, S.A.; Wagner, J.A. Cathepsin K inhibitors: A novel target for osteoporosis therapy. Clin. Pharmacol. Ther. 2008, 83, 172–176. [Google Scholar] [CrossRef]
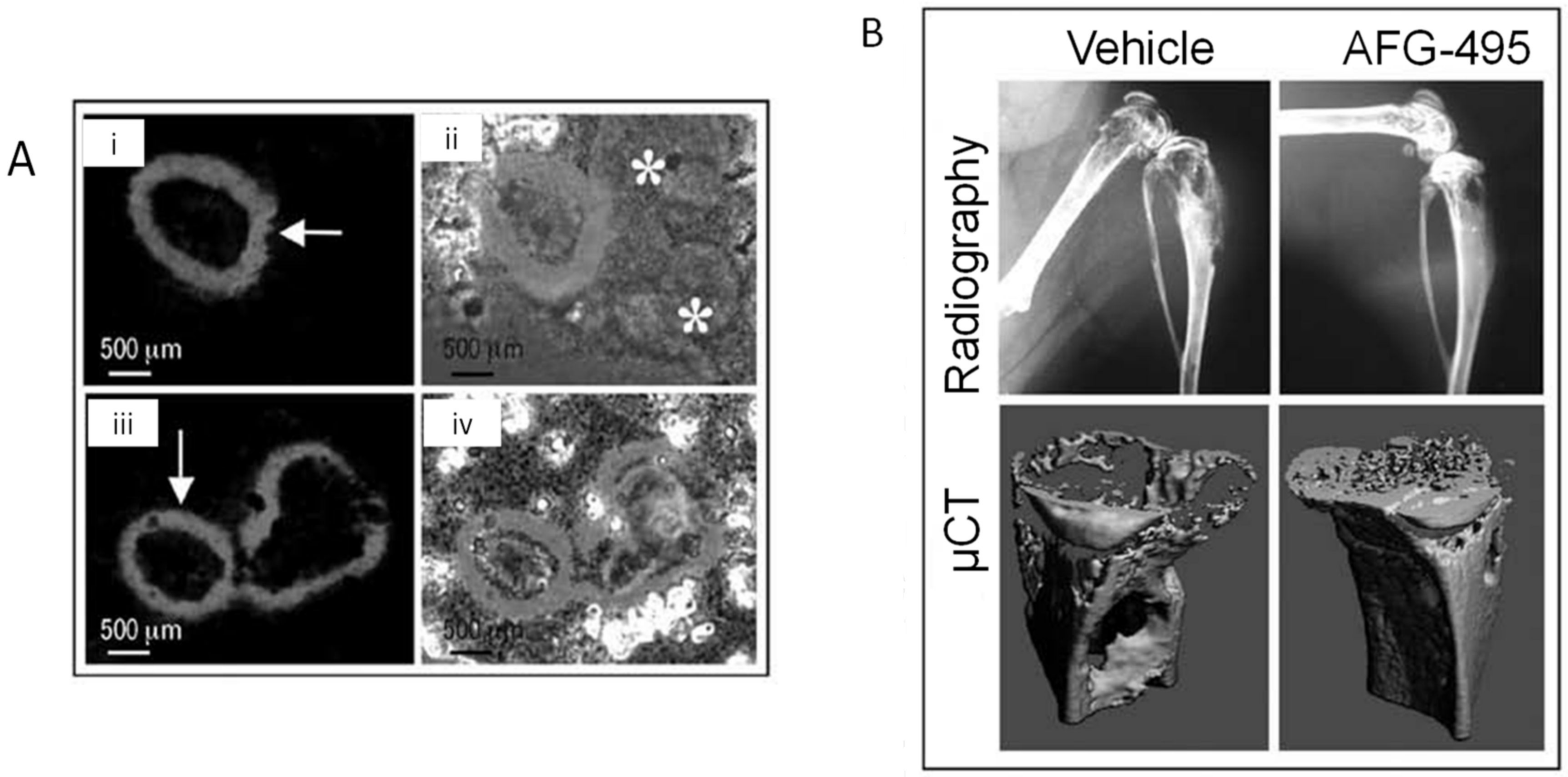
- Duong, L.T.; Wesolowski, G.A.; Leung, P.; Oballa, R.; Pickarski, M. Efficacy of a cathepsin K inhibitor in a preclinical model for prevention and treatment of breast cancer bone metastasis. Mol. Cancer Ther. 2014, 13, 2898–2909. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Sharma, M.C.; Mridha, A.R.; Bakhshi, S. Expression of Cathepsin L in tumor cells and tumor-associated macrophages in patients with Ewing sarcoma family of tumors: A pilot study. Indian J. Pathol. Microbiol. 2015, 58, 170–174. [Google Scholar]
- Brubaker, K.D.; Vessella, R.L.; True, L.D.; Thomas, R.; Corey, E. Cathepsin K mRNA and protein expression in prostate cancer progression. J. Bone Miner. Res. 2003, 18, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Blair, H.C.; Sidonio, R.F.; Friedberg, R.C.; Khan, N.N.; Dong, S.S. Proteinase expression during differentiation of human osteoclasts in vitro. J. Cell. Biochem. 2000, 78, 627–637. [Google Scholar] [CrossRef]
- Joyce, J.A.; Baruch, A.; Chehade, K.; Meyer-Morse, N.; Giraudo, E.; Tsai, F.-Y.; Greenbaum, D.C.; Hager, J.H.; Bogyo, M.; Hanahan, D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004, 5, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Battula, V.L.; Nguyen, K.; Sun, J.; Pitner, M.K.; Yuan, B.; Bartholomeusz, C.; Hail, N.; Andreeff, M. IKK inhibition by BMS-345541 suppresses breast tumorigenesis and metastases by targeting GD2+ cancer stem cells. Oncotarget 2017, 8, 36936–36949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdowska, I. Cysteine proteases as disease markers. Clin. Chim. Acta 2004, 342, 41–69. [Google Scholar] [CrossRef] [PubMed]
- Jedeszko, C.; Sloane, B.F. Cysteine cathepsins in human cancer. Biol. Chem. 2004, 385, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Kohno, K.; Kawamata, T.; Morimitsu, K.; Kuwano, M.; Miyakawa, I. Increased cathepsin L levels in serum in some patients with ovarian cancer: Comparison with CA125 and CA72-4. Gynecol. Oncol. 1995, 56, 357–361. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, D.; Zhang, L.; Su, Q.; Mao, W.; Jiang, C. Expression profile of cathepsins indicates the potential of cathepsins B and D as prognostic factors in breast cancer patients. Oncol. Lett. 2016, 11, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Thomssen, C.; Schmitt, M.; Goretzki, L.; Oppelt, P.; Pache, L.; Dettmar, P.; Jänicke, F.; Graeff, H. Prognostic value of the cysteine proteases cathepsins B and cathepsin L in human breast cancer. Clin. Cancer Res. 1995, 1, 741–746. [Google Scholar]
- Kageshita, T.; Yoshii, A.; Kimura, T.; Maruo, K.; Ono, T.; Himeno, M.; Nishimura, Y. Biochemical and immunohistochemical analysis of cathepsins B, H, L and D in human melanocytic tumours. Arch. Dermatol. Res. 1995, 287, 266–272. [Google Scholar] [CrossRef]
- Campo, E.; Muñoz, J.; Miquel, R.; Palacín, A.; Cardesa, A.; Sloane, B.F.; Emmert-Buck, M.R. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am. J. Pathol. 1994, 145, 301–309. [Google Scholar]
- Werle, B.; Ebert, W.; Klein, W.; Spiess, E. Cathepsin B in tumors, normal tissue and isolated cells from the human lung. Anticancer Res. 1994, 14, 1169–1176. [Google Scholar]
- Schweiger, A.; Staib, A.; Werle, B.; Krasovec, M.; Lah, T.T.; Ebert, W.; Turk, V.; Kos, J. Cysteine proteinase cathepsin H in tumours and sera of lung cancer patients: Relation to prognosis and cigarette smoking. Br. J. Cancer 2000, 82, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, E.; Schlagenhauff, B.; Möhrle, M.; Weber, E.; Klessen, C.; Rassner, G. Activity, expression, and transcription rate of the cathepsins B, D, H, and L in cutaneous malignant melanoma. Cancer 2001, 91, 972–982. [Google Scholar] [CrossRef]
- Lah, T.T.; Calaf, G.; Kalman, E.; Shinde, B.G.; Somers, R.; Estrada, S.; Salero, E.; Russo, J.; Daskal, I. Cathepsins D, B, and L in transformed human breast epithelial cells. Breast Cancer Res Treat. 1996, 39, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.L.; Farré, X.; Nadal, A.; Fernández, E.; Peiró, N.; Sloane, B.F.; Shi, G.-P.; Chapman, H.A.; Campo, E.; Cardesa, A. Expression of cathepsins B and S in the progression of prostate carcinoma. Int. J. Cancer 2001, 95, 51–55. [Google Scholar] [CrossRef]
- Gocheva, V.; Zeng, W.; Ke, D.; Klimstra, D.; Reinheckel, T.; Peters, C.; Hanahan, D.; Joyce, J.A. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006, 20, 543–556. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Sulkowska, M.; Koda, M.; Sulkowski, S. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J. Gastroenterol. 2005, 11, 1251–1266. [Google Scholar] [CrossRef]
- Cairns, R.A.; Khokha, R.; Hill, R.P. Molecular mechanisms of tumor invasion and metastasis: An integrated view. Curr. Mol. Med. 2003, 3, 659–671. [Google Scholar] [CrossRef]
- Sobotič, B.; Vizovišek, M.; Vidmar, R.; Van Damme, P.; Gocheva, V.; Joyce, J.A.; Gevaert, K.; Turk, V.; Turk, B.; Fonović, M. Proteomic Identification of Cysteine Cathepsin Substrates Shed from the Surface of Cancer Cells. Mol. Cell. Proteom. 2015, 14, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Lankelma, J.M.; Voorend, D.M.; Barwari, T.; Koetsveld, J.; Van Der Spek, A.H.; De Porto, A.P.; Van Rooijen, G.; Van Noorden, C.J. Cathepsin L, target in cancer treatment? Life Sci. 2010, 86, 225–233. [Google Scholar] [CrossRef]
- Sawant, A.; Ponnazhagan, S. Myeloid-derived suppressor cells as osteoclast progenitors: A novel target for controlling osteolytic bone metastasis. Cancer Res. 2013, 73, 4606–4610. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, L.; Roato, I. The Impact of Immune System in Regulating Bone Metastasis Formation by Osteotropic Tumors. J. Immunol. Res. 2015, 2015, 143526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gocheva, V.; Wang, H.W.; Gadea, B.B.; Shree, T.; Hunter, K.E.; Garfall, A.L.; Berman, T.; Joyce, J.A. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010, 24, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgorski, I.; Linebaugh, B.E.; Koblinski, J.E.; Rudy, D.L.; Herroon, M.K.; Olive, M.B.; Sloabe, B.F. Bone marrow-derived cathepsin K cleaves SPARC in bone metastasis. Am. J. Pathol. 2009, 175, 1255–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundy, G.R. Mechanisms of bone metastasis. Cancer 1997, 80 (Suppl. 8), 1546–1556. [Google Scholar] [CrossRef]
- Mundy, G.R.; Chen, D.; Zhao, M.; Dallas, S.; Xu, C.; Harris, S. Growth regulatory factors and bone. Rev. Endocr. Metab. Disord. 2001, 2, 105–115. [Google Scholar] [CrossRef]
- Bussard, K.M.; Gay, C.V.; Mastro, A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008, 27, 41–55. [Google Scholar] [CrossRef]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 1998, 139, 4743–4746. [Google Scholar] [CrossRef]
- Rodan, G.A. The development and function of the skeleton and bone metastases. Cancer 2003, 97 (Suppl. 3), 726–732. [Google Scholar] [CrossRef]
- Guise, T.A.; Yin, J.J.; Mohammad, K.S. Role of endothelin-1 in osteoblastic bone metastases. Cancer 2003, 97 (Suppl. 3), 779–784. [Google Scholar] [CrossRef]
- Akhtari, M.; Mansuri, J.; Newman, K.A.; Guise, T.M.; Seth, P. Biology of breast cancer bone metastasis. Cancer Biol. Ther. 2008, 7, 3–9. [Google Scholar] [CrossRef]
- Futakuchi, M.; Nannuru, K.C.; Varney, M.L.; Sadanandam, A.; Nakao, K.; Asai, K.; Shirai, T.; Sato, S.; Singh, R. Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009, 100, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.J.; Nannuru, K.C.; Singh, R.K. Cathepsin G-mediated activation of pro-matrix metalloproteinase 9 at the tumor-bone interface promotes transforming growth factor-beta signaling and bone destruction. Mol. Cancer Res. 2009, 7, 1224–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumminello, F.M.; Flandina, C.; Crescimanno, M.; Leto, G. Circulating cathepsin K and cystatin C in patients with cancer related bone disease: Clinical and therapeutic implications. Biomed. Pharmacother. 2008, 62, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003, 62, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Withana, N.P.; Blum, G.; Sameni, M.; Slaney, C.; Anbalagan, A.; Olive, M.B.; Bidwell, B.N.; Edington, L.; Wang, L.; Moin, K.; et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012, 72, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, Q.; Chen, L. Triptolide induces the cell apoptosis of osteosarcoma cells through the TRAIL pathway. Oncol. Rep. 2016, 36, 1499–1505. [Google Scholar] [CrossRef]
- Garnett, T.O.; Filippova, M.; Duerksen-Hughes, P.J. Bid is cleaved upstream of caspase-8 activation during TRAIL-mediated apoptosis in human osteosarcoma cells. Apoptosis 2007, 12, 1299–1315. [Google Scholar] [CrossRef]
- Nagaraj, N.S.; Vigneswaran, N.; Zacharias, W. Cathepsin B mediates TRAIL-induced apoptosis in oral cancer cells. J. Cancer Res. Clin. Oncol. 2006, 132, 171–183. [Google Scholar] [CrossRef]
- Cirman, T.; Oresić, K.; Mazovec, G.D.; Turk, V.; Reed, J.C.; Myers, R.M.; Salvesen, G.S.; Turk, B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2004, 279, 3578–3587. [Google Scholar] [CrossRef] [Green Version]
- Owa, C.; Messina, M.E.; Halaby, R. Triptolide induces lysosomal-mediated programmed cell death in MCF-7 breast cancer cells. Int. J. Womens Health 2013, 5, 557–569. [Google Scholar] [PubMed] [Green Version]
- Maguire, T.M.; Shering, S.G.; Duggan, C.M.; McDermott, E.W.; O’Higgins, N.J.; Duffy, M.J. High levels of cathepsin B predict poor outcome in patients with breast cancer. Int. J. Biol. Markers 1998, 13, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Foekens, J.A.; Kos, J.; Peters, H.A.; Krasovec, M.; Look, M.P.; Cimerman, N.; Meijer-van Gelder, M.E.; Henzen-Logmans, S.C.; van Putten, W.L.; Klijn, J.G. Prognostic significance of cathepsins B and L in primary human breast cancer. J. Clin. Oncol. 1998, 16, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, I.; Linebaugh, B.E.; Sameni, M.; Jedeszko, C.; Bhagat, S.; Cher, M.L.; Sloane, B.F. Bone microenvironment modulates expression and activity of cathepsin B in prostate cancer. Neoplasia 2005, 7, 207–223. [Google Scholar] [CrossRef] [Green Version]
- Arkona, C.; Wiederanders, B. Expression, subcellular distribution and plasma membrane binding of cathepsin B and gelatinases in bone metastatic tissue. Biol. Chem. 1996, 377, 695–702. [Google Scholar] [CrossRef]
- Verdoes, M.; Oresic Bender, K.; Segal, E.; van der Linden, W.A.; Syed, S.; Withana, N.P.; Sanman, L.E.; Bogyo, M. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J. Am. Chem. Soc. 2013, 135, 14726–14730. [Google Scholar] [CrossRef] [Green Version]
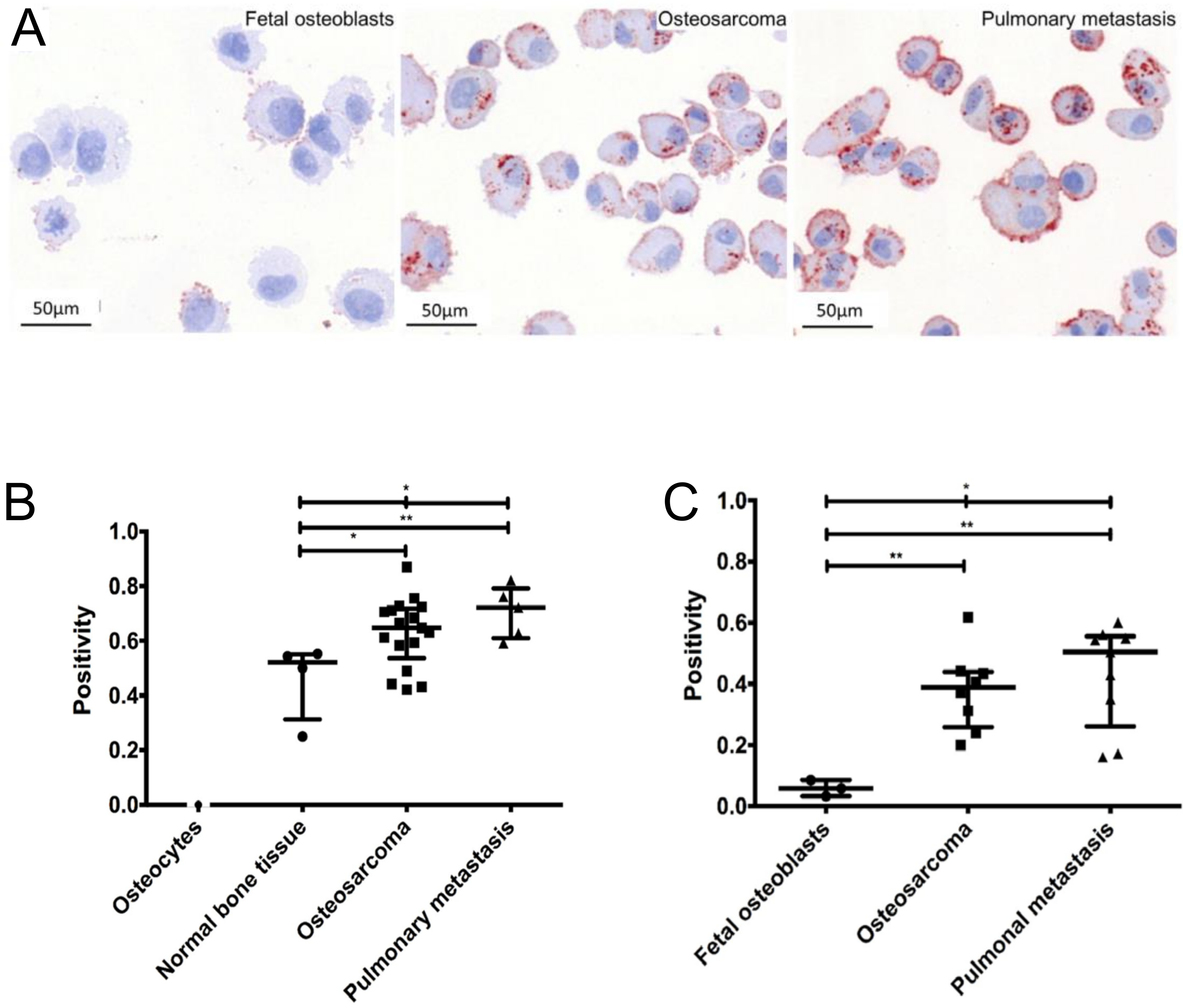
- Gemoll, T.; Epping, F.; Heinrich, L.; Fritzsche, B.; Roblick, U.J.; Szymczak, S.; Hartwig, S.; Depping, R.; Bruch, H.; Thorns, C.; et al. Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget 2015, 6, 16517–16526. [Google Scholar] [CrossRef] [Green Version]
- Spreafico, A.; Frediani, B.; Capperucci, C.; Chellini, F.; Paffetti, A.; D’Ambrosio, C.; Bernardini, G.; Mini, R.; Collodel, G.; Scaloni, A.; et al. A proteomic study on human osteoblastic cells proliferation and differentiation. Proteomics 2006, 6, 3520–3532. [Google Scholar] [CrossRef]
- Rochefort, H. Cathepsin D in breast cancer: A tissue marker associated with metastasis. Eur. J. Cancer 1992, 28A, 1780–1783. [Google Scholar] [CrossRef]
- Pujol, P.; Maudelonde, T.; Daures, J.P.; Rouanet, P.; Brouillet, J.P.; Pujol, H.; Rochefort, H. A prospective study of the prognostic value of cathepsin D levels in breast cancer cytosol. Cancer 1993, 71, 2006–2012. [Google Scholar] [CrossRef]
- Schultz, D.C.; Bazel, S.; Wright, L.M.; Tucker, S.; Lange, M.K.; Tachovsky, T.; Longo, S.; Niedbala, S.; Alhadeff, J.A. Western blotting and enzymatic activity analysis of cathepsin D in breast tissue and sera of patients with breast cancer and benign breast disease and of normal controls. Cancer Res. 1994, 54, 48–54. [Google Scholar] [PubMed]
- Ravdin, P.M. Evaluation of cathepsin D as a prognostic factor in breast cancer. Breast Cancer Res. Treat. 1993, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Solomayer, E.F.; Diel, I.J.; Meyberg, G.C.; Gollan, C.; Bode, S.; Wallwiener, D.; Bastert, G. Prognostic relevance of cathepsin D detection in micrometastatic cells in the bone marrow of patients with primary breast cancer. Breast Cancer Res. Treat. 1998, 49, 145–154. [Google Scholar] [CrossRef]
- Arai, K.; Sakamoto, R.; Kubota, D.; Kondo, T. Proteomic approach toward molecular backgrounds of drug resistance of osteosarcoma cells in spheroid culture system. Proteomics 2013, 13, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, V.; Muth, D.; Sagulenko, E.; Paffhausen, T.; Schwab, M.; Westermann, F. Cathepsin D protects human neuroblastoma cells from doxorubicin-induced cell death. Carcinogenesis 2008, 29, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Chen, C.M.; Yu, K.D.; Yang, W.T.; Shao, Z.M. A prognostic model to predict outcome of patients failing to achieve pathological complete response after anthracycline-containing neoadjuvant chemotherapy for breast cancer. J. Surg. Oncol. 2012, 105, 577–585. [Google Scholar] [CrossRef]
- Avnet, S.; Longhi, A.; Salerno, M.; Halleen, J.M.; Perut, F.; Granchi, D.; Ferrari, S.; Bertoni, F.; Giunti, A.; Baldini, N. Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int. J. Oncol. 2008, 33, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Wilson, T.J.; Nannuru, K.C.; Futakuchi, M.; Sadanandam, A.; Singh, R.K. Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-kappaB ligand. Cancer Res. 2008, 68, 5803–5811. [Google Scholar] [CrossRef] [Green Version]
- Lü, J.; Qian, J.; Keppler, D.; Cardoso, W.V. Cathespin H is an Fgf10 target involved in Bmp4 degradation during lung branching morphogenesis. J. Biol. Chem. 2007, 282, 22176–22184. [Google Scholar] [CrossRef] [Green Version]
- Verbovšek, U.; Van Noorden, C.J.; Lah, T.T. Complexity of cancer protease biology: Cathepsin K expression and function in cancer progression. Semin Cancer Biol. 2015, 35, 71–84. [Google Scholar] [CrossRef]
- Blouin, S.; Baslé, M.F.; Chappard, D. Interactions between microenvironment and cancer cells in two animal models of bone metastasis. Br. J. Cancer 2008, 98, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Shi, G.P.; Heller, M.; Weremowicz, S.; Morton, C.; Desnick, R.J.; Chapman, H.A. Structure and chromosomal assignment of the human cathepsin K gene. Genomics 1997, 41, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.A.; Van Horn, S.; Drake, F.H.; Gowen, M.; Debouck, C. Genomic organization and chromosome localization of the human cathepsin K gene (CTSK). Genomics 1997, 41, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Chen, W. Characterization of mouse cathepsin K gene, the gene promoter, and the gene expression. J. Bone Miner. Res. 1999, 14, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Pickarski, M.; Wesolowski, G.A.; Duong, L.T. Anti-resorptive and potential anti-invasion effects of a cathepsin K inhibitor in the treatment of breast cancer-induced bone metastasis. Cancer Treat. Rev. 2008, 34 (Suppl. 1), S58. [Google Scholar] [CrossRef]
- Munari, E.; Cima, L.; Massari, F.; Bertoldo, F.; Porcaro, A.B.; Caliò, A.; Riva, G.; Ciocchetta, E.; Ciccarese, C.; Modena, A.; et al. Cathepsin K expression in castration-resistant prostate carcinoma: A therapeutical target for patients at risk for bone metastases. Int. J. Biol. Markers 2017, 32, e243–e247. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Wang, F.; Chen, Q.; Dai, J.; Escara-Wilke, J.; Keller, E.T.; Zimmerman, J.; Hona, N.; Lu, Y.; Zhang, J. Targeting cathepsin K diminishes prostate cancer establishment and growth in murine bone. J. Cancer Res. Clin. Oncol. 2019, 145, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Katunuma, N.; Tsuge, H.; Nukatsuka, M.; Asao, T.; Fukushima, M. Structure-based design of specific cathepsin inhibitors and their application to protection of bone metastases of cancer cells. Arch. Biochem. Biophys. 2002, 397, 305–311. [Google Scholar] [CrossRef]
- Yoneda, T. Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur. J. Cancer 1998, 34, 240–245. [Google Scholar] [CrossRef]
- Furuyama, N.; Fujisawa, Y. Distinct roles of cathepsin K and cathepsin L in osteoclastic bone resorption. Endocr. Res. 2000, 26, 189–204. [Google Scholar] [CrossRef]
- Furuyama, N.; Fujisawa, Y. Regulation of collagenolytic protease secretion through c-Src in osteoclasts. Biochem. Biophys. Res. Commun. 2000, 272, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, F.; Yamashita, U.; Tanaka, Y.; Watanaba, K.; Sato, K.; Haratake, J.; Fujihira, T.; Oda, S.; Eto, S. Production of bone-resorbing activity corresponding to interleukin-1 alpha by adult T-cell leukemia cells in humans. Cancer Res. 1988, 48, 4284–4287. [Google Scholar] [PubMed]
- Jay, P.R.; Centrella, M.; Lorenzo, J.; Bruce, A.G.; Horowitz, M.C. Oncostatin-M: A new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology 1996, 137, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, E.M.; Horowitz, M.C.; Lavish, S.A. Stimulation by parathyroid hormone of interleukin-6 and leukemia inhibitory factor expression in osteoblasts is an immediate-early gene response induced by cAMP signal transduction. J. Biol. Chem. 1996, 271, 10984–10989. [Google Scholar] [CrossRef] [Green Version]
- Damiens, C.; Grimaud, E.; Rousselle, A.V.; Charrier, C.; Fortun, Y.; Heymann, D.; Padrines, M. Cysteine protease production by human osteosarcoma cells (MG63, SAOS2) and its modulation by soluble factors. Cytokine 2000, 12, 539–542. [Google Scholar] [CrossRef]
- Park, I.C.; Lee, S.Y.; Jeon, D.G.; Lee, J.S.; Hwang, C.S.; Hwang, Y.G.; Lee, S.H.; Hong, W.S.; Hong, S.I. Enhanced expression of cathepsin L in metastatic bone tumors. J. Korean Med. Sci. 1996, 11, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Bincoletto, C.; Tersariol, I.L.; Oliveira, C.R.; Dreher, S.; Fausto, D.M.; Soufen, M.A.; Nascimento, F.D.; Caires, A.C.F. Chiral cyclopalladated complexes derived from N,N-dimethyl-1-phenethylamine with bridging bis(diphenylphosphine)ferrocene ligand as inhibitors of the cathepsin B activity and as antitumoral agents. Bioorg. Med. Chem. 2005, 13, 3047–3055. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Oliveira, C.R.; Nascimento, F.D.; Smith, M.C.; Fausto, D.M.; Soufen, M.A.; Sena, E.; Araújo, R.C.; Tersariol, I.L.S.; Bincoletto, C.; et al. Biphosphinic palladacycle complex mediates lysosomal-membrane permeabilization and cell death in K562 leukaemia cells. Eur. J. Pharmacol. 2006, 542, 37–47. [Google Scholar] [CrossRef]
- Bechara, A.; Barbosa, C.M.; Paredes-Gamero, E.J.; Garcia, D.M.; Silva, L.S.; Matsuo, A.L.; Nascimento, F.D.; Rodrigues, E.G.; Caires, A.C.F.; Smaili, S.S.; et al. Palladacycle (BPC) antitumour activity against resistant and metastatic cell lines: The relationship with cytosolic calcium mobilisation and cathepsin B activity. Eur. J. Med. Chem. 2014, 79, 24–33. [Google Scholar] [CrossRef]
- Krueger, S.; Kellner, U.; Buehling, F.; Roessner, A. Cathepsin L antisense oligonucleotides in a human osteosarcoma cell line: Effects on the invasive phenotype. Cancer Gene Ther. 2001, 8, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.; Friedrichs, K.; Noel, D.; Pintér, T.; Van Belle, S.; Vorobiof, D.; Duarte, R.; Gil Gil, M.; Bodrogi, I.; Murray, E.; et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J. Clin. Oncol. 1999, 17, 2341–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Chu, F.; Chou, P.M.; Gallati, C.; Dier, U.; Mirkin, B.L.; Mousa, S.A.; Rebbaa, A. Cathepsin L inhibition suppresses drug resistance in vitro and in vivo: A putative mechanism. Am. J. Physiol. Cell. Physiol. 2009, 296, C65–C74. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chou, P.M.; Mirkin, B.L.; Rebbaa, A. Senescence-initiated reversal of drug resistance: Specific role of cathepsin L. Cancer Res. 2004, 64, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selak, M.A.; Chignard, M.; Smith, J.B. Cathepsin G is a strong platelet agonist released by neutrophils. Biochem. J. 1988, 251, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewiecki, E.M. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs 2009, 12, 799–809. [Google Scholar] [PubMed]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Léger, S.; LeRiche, T.; et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef]
- Desmarais, S.; Massé, F.; Percival, M.D. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: A comparison of available tools. Biol. Chem. 2009, 390, 941–948. [Google Scholar] [CrossRef]
- Jensen, A.B.; Wynne, C.; Ramirez, G.; He, W.; Song, Y.; Berd, Y.; Wang, H.; Mehta, A.; Lombardi, A. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: Results of a 4-week, double-blind, randomized, controlled trial. Clin. Breast Cancer 2010, 10, 452–458. [Google Scholar] [CrossRef]
- Peroni, A.; Zini, A.; Braga, V.; Colato, C.; Adami, S.; Girolomoni, G. Drug-induced morphea: Report of a case induced by balicatib and review of the literature. J. Am. Acad. Dermatol. 2008, 59, 125–129. [Google Scholar] [CrossRef]
- Mullard, A. Merck &Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov. 2016, 15, 669. [Google Scholar]
- Althoff, E. Novartis R&D Update Highlights Industry Leading Development Pipeline Including Potential Blockbusters and Advanced Therapy Platforms; Novartis Media Relations: London, UK, 2018. [Google Scholar]
- Palmer, J.T.; Bryant, C.; Wang, D.X.; Davis, D.E.; Setti, E.L.; Rydzewski, R.M.; Venkatraman, S.; Tian, Z.Q.; Burrill, L.C.; Mendonca, R.V.; et al. Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J. Med. Chem. 2005, 48, 7520–7534. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, C.; Bellahcène, A.; Bonnelye, E.; Gasser, J.A.; Castronovo, V.; Green, J.; Zimmermann, J.; Clézardin, P. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007, 67, 9894–9902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, L.J.; Smith, B.A.; Smith, B.N.; Loyd, Q.; Nagappan, P.; McKeithen, D.; Wilder, C.L.; Platt, M.O.; Hudson, T.; Odero-Marah, V.A. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis 2015, 36, 1019–1027. [Google Scholar] [CrossRef]
- Odero-Marah, V.A.; Wang, R.; Chu, G.; Zayzafoon, M.; Xu, J.; Shi, C.; Marshall, F.F.; Zhau, H.E.; Chung, L.W.K. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008, 18, 858–870. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.D.; Chavarria, G.E.; Charlton-Sevcik, A.K.; Yoo, G.K.; Song, J.; Strecker, T.E.; Siim, B.G.; Chaplin, D.J.; Trawick, M.L.; Pinney, K.G. Functionalized benzophenone, thiophene, pyridine, and fluorene thiosemicarbazone derivatives as inhibitors of cathepsin L. Bioorg. Med. Chem. Lett. 2010, 20, 6610–6615. [Google Scholar] [CrossRef]
- Kishore Kumar, G.D.; Chavarria, G.E.; Charlton-Sevcik, A.K.; Arispe, W.M.; Macdonough, M.T.; Strecker, T.E.; Chen, S.; Siim, B.G.; Chapli, D.J.; Trawick, M.L.; et al. Design, synthesis, and biological evaluation of potent thiosemicarbazone based cathepsin L inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1415–1419. [Google Scholar] [CrossRef]
- Sudhan, D.R.; Rabaglino, M.B.; Wood, C.E.; Siemann, D.W. Cathepsin L in tumor angiogenesis and its therapeutic intervention by the small molecule inhibitor KGP94. Clin. Exp. Metastasis 2016, 33, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clin. Exp. Metastasis 2013, 30, 891–902. [Google Scholar] [CrossRef] [Green Version]



| Cathepsin Target | Agent | Study Type | Target Approach | Effects | Side Effects |
|---|---|---|---|---|---|
| CatB | CA074Me | Preclinical | SMI |
| -- |
| BPC | Preclinical | SMI |
| -- | |
| CatG | TPCK | Preclinical | SMI |
| -- |
| CatK | Genetic inhibition | Preclinical | CatK null mice |
| -- |
| AFG-495 | Preclinical | SMI |
| -- | |
| CKI | Preclinical | SMI |
| -- | |
| L-235 |
| -- | |||
| Odanacatib | Preclinical/Clinical | SMI |
| -Increase risk of stroke | |
| -Skin rashes | |||||
| CatL | Genetic inhibition | Preclinical | Antisense oligonucleotides |
| -- |
| iCL | Preclinical | SMI |
| -- | |
| Muscadine grape skin extract | Preclinical |
| -- | ||
| CLIK-148 | Preclinical | SMI |
| -- | |
| CatB/CatK | E64 | Preclinical | SMI |
| -- |
| CatK/CatL | KGP94 | Preclinical | SMI |
| -- |
| Pan-cathepsin inhibitor | JMP-OEt | SMI |
| -- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasanya, H.O.; Siemann, D.W. The Role of Cathepsins in the Growth of Primary and Secondary Neoplasia in the Bone. Osteology 2021, 1, 3-28. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1010002
Fasanya HO, Siemann DW. The Role of Cathepsins in the Growth of Primary and Secondary Neoplasia in the Bone. Osteology. 2021; 1(1):3-28. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1010002
Chicago/Turabian StyleFasanya, Henrietta O., and Dietmar W. Siemann. 2021. "The Role of Cathepsins in the Growth of Primary and Secondary Neoplasia in the Bone" Osteology 1, no. 1: 3-28. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1010002





