Ecological Study of Fractures in Paediatric Melanesian Communities with Varying Endemic Environmental Fluoride Exposure
Abstract
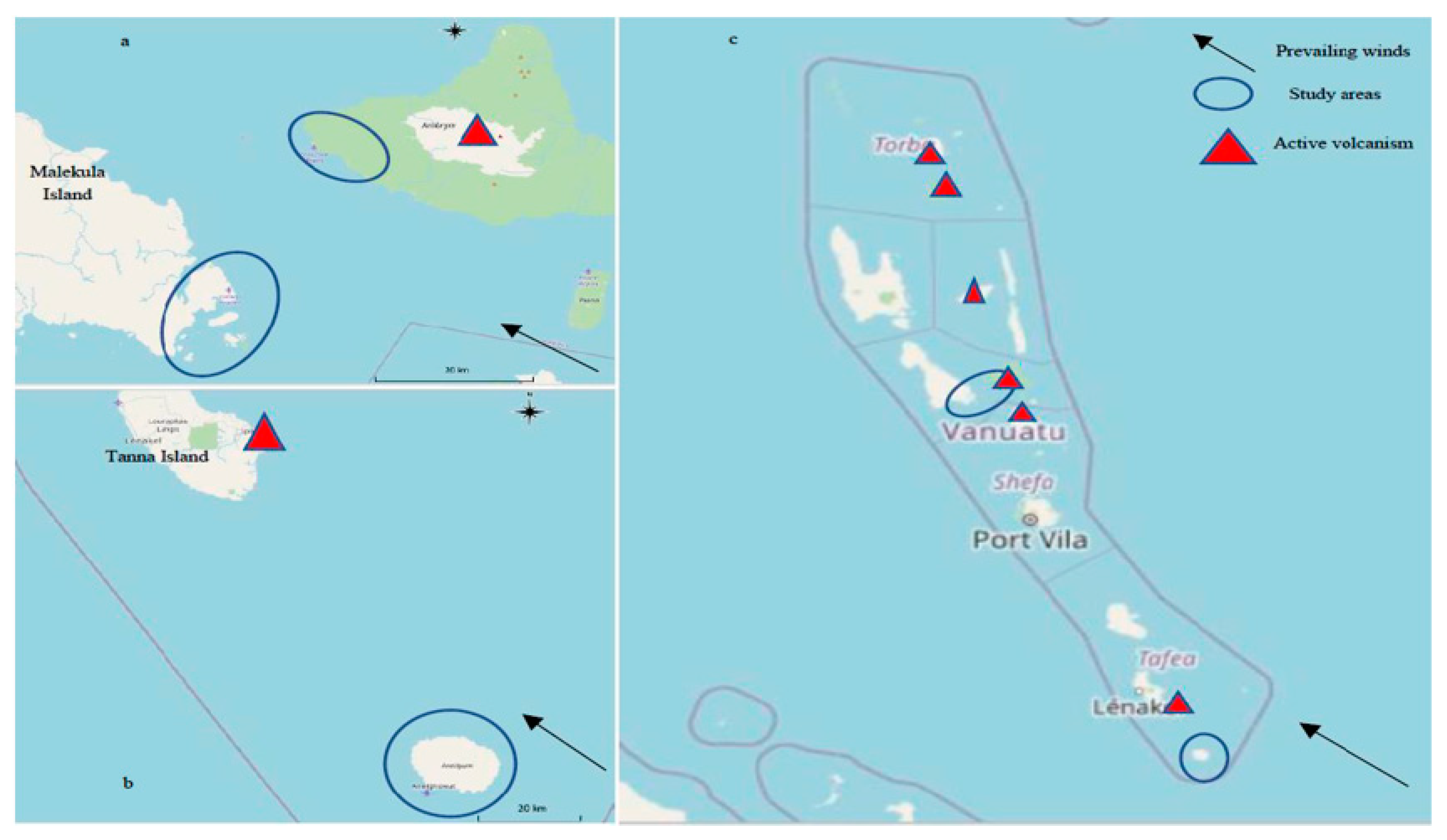
:1. Introduction
2. Methods
3. Clinical Assessment
4. Fluoride Measurement
5. Statistical Methods
6. Results
6.1. Fracture Rates in Areas of Low, Medium and High Endemic Fluorosis
6.2. Location of Fracture
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Clynes, M.; Harvey, N.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Bhadada, S.; Ebeling, P.; Gilchrist, N.; Khan, A.; Halbout, P.; Lekamwasam, S.; Lyubomirsky, G.; Mitchell, P.; Nguyen, T.; et al. IQ driving QI: The Asia Pacific Consortium on Osteoporosis (APCO): An innovative and collaborative initiative to improve osteoporosis care in the Asia Pacific. Osteoporos. Int. 2020, 31, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, M.; Malathi, N.; Ramesh, K.; Aruna, R.M.; Kuruvilla, S. Comparative Evaluation of Dental and Skeletal Fluorosis in an Endemic Fluorosed District, Salem, Tamil Nadu. J. Pharm. Bioallied Sci. 2017, 9, S88–S91. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.J.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary Fluoride Intake and Associated Skeletal and Dental Fluorosis in School Age Children in Rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Ye, Q.; Chen, W.; Zhao, Z.; Li, L.; Lin, P. Study of the relationship between the lifestyle of residents residing in fluorosis endemic areas and adult skeletal fluorosis. Environ. Toxicol. Pharmacol. 2015, 40, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, H.G.; Heslop, P.; Kisima, J.; Gray, W.K.; Ndossi, G.; Maguire, A.; Walker, R.W. Prevalence and aetiology of juvenile skeletal fluorosis in the south-west of the Hai district, Tanzania—A community-based prevalence and case-control study. Trop. Med. Int. Health 2012, 18, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Melaku, Z.; Assefa, G.; Enqusilassie, F.; Bjorvatn, K.; Tekle-Haimanot, R. Epidemiology of skeletal fluorosis in Wonji Shoa Sugar Estate, Wonji, Ethiopia: A community based survey. Ethiop. Med. J. 2012, 50, 307–313. [Google Scholar] [PubMed]
- Oruc, N. Occurrence and problems of high fluoride waters in Turkey: An overview. Environ. Geochem. Health 2008, 30, 315–323. [Google Scholar] [CrossRef]
- McGill, P.E. Endemic fluorosis. Bailliere Clin. Rheumatol. 1995, 9, 75–81. [Google Scholar] [CrossRef]
- Teotia, M.; Teotia, S.P.S.; Kunwar, K.B. Endemic skeletal fluorosis. Arch. Dis. Child. 1971, 46, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.M.; Wig, K.L. Chronic Endemic Fluorosis (With Bone Affections) in the Punjab. Indian Med Gaz. 1945, 80, 429–433. [Google Scholar]
- Jolly, S.S.; Singh, B.M.; Mathur, O.C.; Malhotra, K.C. Epidemiological, Clinical, and Biochemical Study of Endemic Dental and Skeletal Fluorosis in Punjab. Br. Med. J. 1968, 4, 427–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, S.; Sing, B.; Mathur, O. Endemic fluorosis in Punjab (India). Am. J. Med. 1969, 47, 553–563. [Google Scholar] [CrossRef]
- Petrone, P.P.; Giordano, M.; Giustino, S.; Guarino, F.M. Enduring Fluoride Health Hazard for the Vesuvius Area Population: The Case of AD 79 Herculaneum. PLoS ONE 2011, 6, e21085. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, W.M.; Smedley, P.L. Fluoride in natural waters. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer Publishing: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Stewart, C.; Damby, D.E.; Tomašek, I.; Horwell, C.J.; Plumlee, G.S.; Armienta, M.A.; Hinojosa, M.G.R.; Appleby, M.; Delmelle, P.; Cronin, S.; et al. Assessment of leachable elements in volcanic ashfall: A review and evaluation of a standardized protocol for ash hazard characterization. J. Volcanol. Geotherm. Res. 2020, 392, 106756. [Google Scholar] [CrossRef]
- d’Alessandro, W. Human fluorosis related to volcanic activity: A review. WIT Trans. Biomed. Health 2006, 10, 21–30. [Google Scholar]
- Cronin, S.J.; Sharp, D.S. Environmental impacts on health from continuous volcanic activity at Yasur (Tanna) and Ambrym, Vanuatu. Int. J. Environ. Health Res. 2002, 12, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Carn, S.A.; Fioletov, V.E.; McLinden, C.; Li, C.; Krotkov, N. A decade of global volcanic SO2 emissions measured from space. Sci. Rep. 2017, 7, srep44095. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Murray, J.J.; Fairpo, C.G. Life-long benefits of fluoride in drinking water. Br. Dent. J. 1973, 134, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, Y.; Gilula, L.A.; Wilson, A.J. Endemic fluorosis of the skeleton: Radiographic features in 127 patients. Am. J. Roentgenol. 1994, 162, 93–98. [Google Scholar] [CrossRef]
- Teotia, S.P.S.; Teotia, M. Secondary Hyperparathyroidism in Patients with Endemic Skeletal Fluorosis. Br. Med. J. 1973, 1, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Köroğlu, B.K.; Ersoy, I.H.; Köroğlu, M.; Balkarli, A.; Ersoy, S.; Varol, S.; Tamer, M.N. Serum Parathyroid Hormone Levels in Chronic Endemic Fluorosis. Biol. Trace Element Res. 2010, 143, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Prevalence of fluorosis in an endemic village in central India. Trop. Dr. 2010, 40, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.S. Indexes for Measuring Dental Fluorosis. J. Public Health Dent. 1986, 46, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.; Dennison, E.; Faibairn-Dunlop, P.; Stewart, P. Paediatric Oral Health Burden in Vanuatu Influenced by Volcanogenic Fluoride. 2019. Available online: https://iadr.abstractarchives.com/abstract/19iags-3171751/paediatric-oral-health-burden-in-vanuatu-influenced-by-volcanogenic-fluoride (accessed on 27 July 2021).
- No Hospital. Available online: http://documents1.worldbank.org/curated/en/802001468125696716/pdf/895050WP0Healt00Box385284B00PUBLIC0.pdf (accessed on 27 July 2021).
- NOHS. National Oral Health Survey of Vanuatu (NOHS) 2018. Report. Ministry of Health Vanuatu, Sailing Ministries, PVC Health Vanuatu. 2017. Available online: https://msm.org.au/launching-of-the-national-oral-health-survey/ (accessed on 27 July 2021).
- Levy, S.M.; Eichenberger-Gilmore, J.M.; Warren, J.J.; Kavand, G.; Letuchy, E.; Broffitt, B.; Marshall, T.A.; Burns, T.L.; Janz, K.F.; Pauley, C.; et al. Associations of fluoride intake with children’s cortical bone mineral and strength measures at age 11. J. Public Health Dent. 2018, 78, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Akdogan, M.; Tamer, N.; Oral, B. Bone Mineral Density of the Spine and Femur in Early Postmenopausal Turkish Women with Endemic Skeletal Fluorosis. Calcif. Tissue Int. 2003, 72, 689–693. [Google Scholar] [CrossRef]
- Teotia, M.; Teotia, S.P.; Singh, K.P. Endemic chronic fluoride toxicity and dietary calcium deficiency interaction syndromes of metabolic bone disease and deformities in India: Year 2000. Indian J. Pediatr. 1998, 65, 371–381. [Google Scholar] [CrossRef]
- Shashi, A.; Kumar, M.; Bhardwaj, M. Incidence of skeletal deformities in endemic fluorosis. Trop. Dr. 2008, 38, 231–233. [Google Scholar]

| Aneityum (Low Fluoride) N = 192 | Lamap (Medium Fluoride) N = 524 | AMBRYM (High Fluoride) N = 225 | ||||
|---|---|---|---|---|---|---|
| Boys N = 118 | Girls N = 74 | Boys N = 255 | Girls N = 269 | Boys N = 110 | Girls N = 115 | |
| Age (SD) years | 8.61 (SD 1.44) | 7.77 (1.15) | 10.3 (3.13) | 10.6 (3.73) | 9.53 (3.03) | 10.15 (3.32) |
| Reported fracture rates (n, %, any site) | 6 4.6% | 4 5.4% | 38 14.9% | 42 15.6% | 5 4.5% | 3 2.6% |
| Boys | Girls | |
|---|---|---|
| Skull | 1 (2%) | 1 (2.0%) |
| UPPER LIMB (n = 57) | ||
| Humerus | 1 (2%) | 4 (8.2%) |
| Forearm | 18 (36.7%) | 15 (30.6%) |
| Wrist | 6 (12.2%) | 6 (12.2%) |
| Hand | 1 (2%) | 6 (12.2%) |
| LOWER LIMB (n = 18) | ||
| Pelvis | 1 (2%) | 1 (2%) |
| Patella | 1 (2%) | 0 (0%) |
| Lower leg | 5 (10.2%) | 3 (6.1%) |
| Ankle | 3 (6.1%) | 4 (8.2%) |
| Unspecified | 12 (24.5%) | 9 (18.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizabeth, W.; Elmansouri, A.; Ross, R.; Clynes, M.; Tangis, J.; Stewart, C.; Dennison, E.M. Ecological Study of Fractures in Paediatric Melanesian Communities with Varying Endemic Environmental Fluoride Exposure. Osteology 2021, 1, 132-140. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1030014
Elizabeth W, Elmansouri A, Ross R, Clynes M, Tangis J, Stewart C, Dennison EM. Ecological Study of Fractures in Paediatric Melanesian Communities with Varying Endemic Environmental Fluoride Exposure. Osteology. 2021; 1(3):132-140. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1030014
Chicago/Turabian StyleElizabeth, Webb, Ahmad Elmansouri, Rebecca Ross, Michael Clynes, Jenny Tangis, Carol Stewart, and Elaine M. Dennison. 2021. "Ecological Study of Fractures in Paediatric Melanesian Communities with Varying Endemic Environmental Fluoride Exposure" Osteology 1, no. 3: 132-140. https://0-doi-org.brum.beds.ac.uk/10.3390/osteology1030014






