Empagliflozin Use and Fournier’s Gangrene: Case Report and Systematic Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Type of Studies, Inclusion and Exclusion Criteria
3. Results
3.1. Main Outcomes
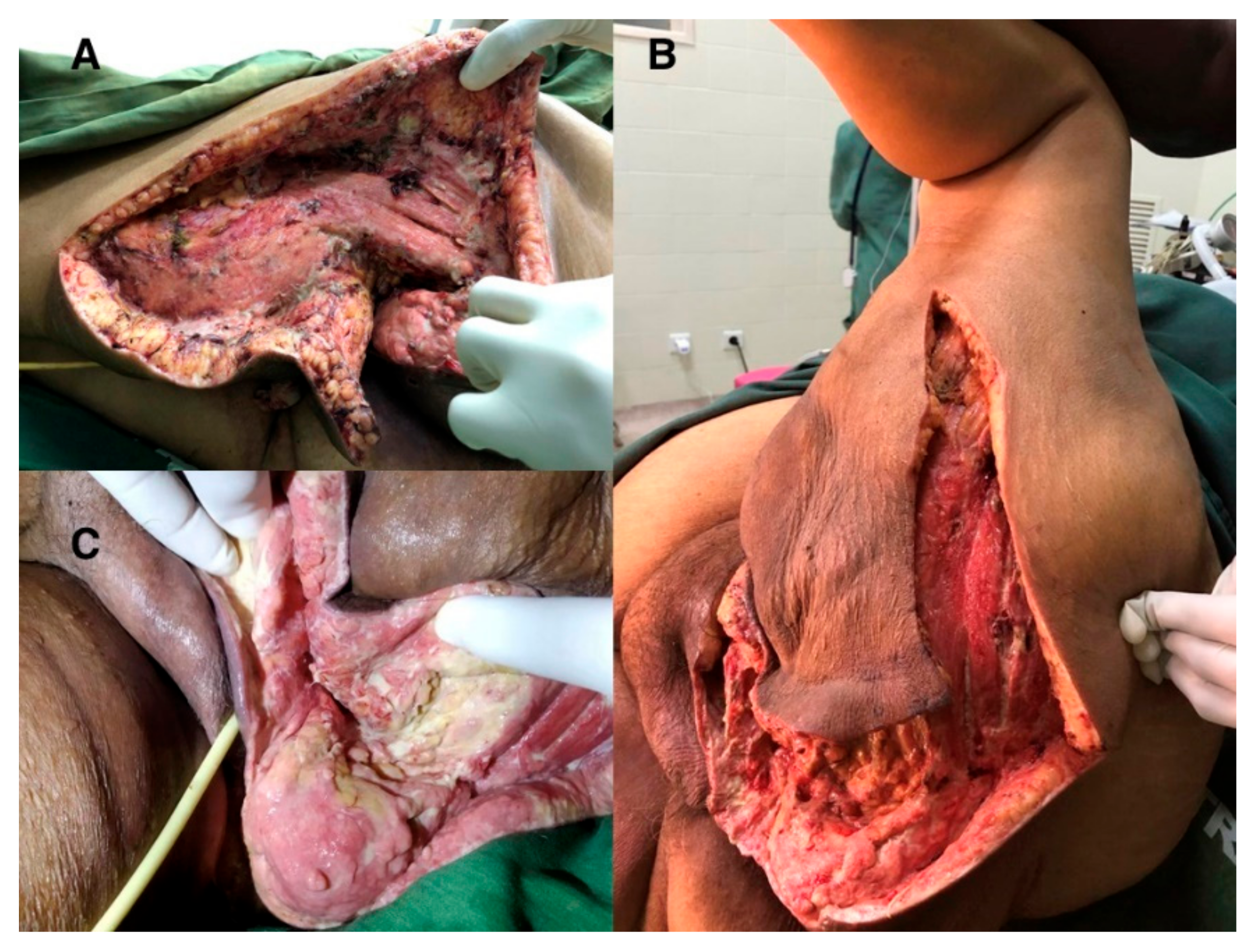
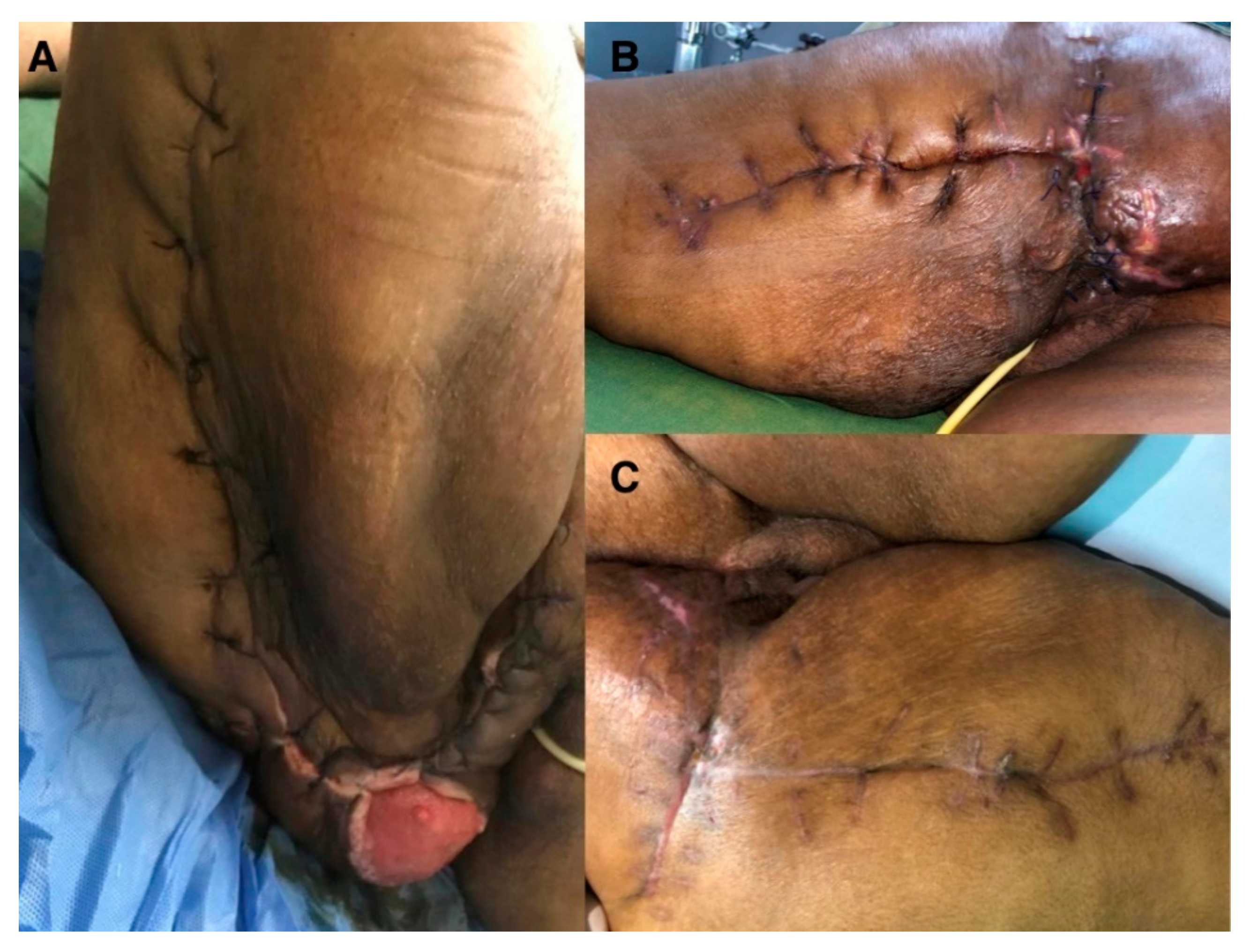
3.2. Case Report
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hakkarainen, T.W.; Kopari, N.M.; Pham, T.N.; Evans, H.L. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr. Probl. Surg. 2014, 51, 344–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadler, T.; Huey, S.; Hunt, K. Recognizing Fournier’s Gangrene in the Emergency Department. Adv. Emerg. Nurs. J. 2019, 41, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.M.; Su, Y.J.; Lai, Y.C. The evaluation of microbiology and prognosis of Fournier’s gangrene in past five years. Springerplus 2015, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernyadyev, S.A.; Ufimtseva, M.A.; Vishnevskaya, I.F.; Bochkarev, Y.M.; Ushakov, A.A.; Beresneva, T.A.; Galimzyanov, F.V.; Khodakov, V.V. Fournier’s Gangrene: Literature Review and Clinical Cases. Urol. Int. 2018, 101, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Montrief, T.; Long, B.; Koyfman, A.; Auerbach, J. Fournier Gangrene: A Review for Emergency Clinicians. J. Emerg. Med. 2019, 57, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, D.S. Risk of Rare, Serious Genital Infection from some Diabetes Drugs. Am. J. Nurs. 2018, 118, 23. [Google Scholar] [CrossRef] [PubMed]
- Watada, H.; Yamauchi, T.; Yamamoto, F.; Taniguchi, A.; Yarush, L.; Heilmann, C.; Yasui, A. Safety and tolerability of empagliflozin and linagliptin combination therapy in patients with type 2 diabetes mellitus: A pooled analysis of data from five randomized, controlled clinical trials. Expert Opin. Drug Saf. 2020, 19, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Berso–Matcha, S.J.; Chamberlain, C.; Cao, C.; Kortepeter, C.; Chong, W.H. Fournier gangrene associated with sodium-glucose cotrans- porter-2 inhibitors. Ann. Intern. Med. 2019, 170, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Yakame, N.K.; Aoki, H.; Yamakawa, T.; Kondo, N.I. Fournier’s Gangrene in a Patient with Type 2 Diabetes Mellitus Treated with Empagliflozin: A Case Report. Drug Saf. Case Rep. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandra Pai, R.P.; Kangath, R.V. Bilateral gangrene of fingers in a patient on empagliflozin: First case report. World J. Diabetes 2019, 10, 133–136. [Google Scholar] [PubMed]
- Kumar, S.; Costello, A.J.; Colman, P.G. Fournier’s gangrene in a man on empagliflozin for treatment of Type 2 diabetes. Diabet Med. 2017, 34, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Elshimy, G.; Correa, R.; Alsayed, M.; Jyothinagaram, S. Early Presentation of a Rare Complication of Sodium-Glucose Cotransporter-2 Inhibitors 10 Days after Initiation: Case Report and Literature Review. Cureus 2019, 11, e5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, M.; de León, A.C.; Pizzol, D.; Seni, A.H.A.; Trott, M.; Carrie, A.M.; Ilie, P.-C.; Veronese, N.; Smith, L. Empagliflozin Use and Fournier’s Gangrene: Case Report and Systematic Literature Review. Surgeries 2021, 2, 174-179. https://0-doi-org.brum.beds.ac.uk/10.3390/surgeries2020018
Antunes M, de León AC, Pizzol D, Seni AHA, Trott M, Carrie AM, Ilie P-C, Veronese N, Smith L. Empagliflozin Use and Fournier’s Gangrene: Case Report and Systematic Literature Review. Surgeries. 2021; 2(2):174-179. https://0-doi-org.brum.beds.ac.uk/10.3390/surgeries2020018
Chicago/Turabian StyleAntunes, Mario, Antonio Cabrera de León, Damiano Pizzol, Amir Hussein Abubacar Seni, Mike Trott, Anne Marie Carrie, Petre-Cristian Ilie, Nicola Veronese, and Lee Smith. 2021. "Empagliflozin Use and Fournier’s Gangrene: Case Report and Systematic Literature Review" Surgeries 2, no. 2: 174-179. https://0-doi-org.brum.beds.ac.uk/10.3390/surgeries2020018





