Normalized Difference Vegetation Index Determination in Urban Areas by Full-Spectrum Photography
Abstract
:1. Introduction
2. Experimental Section
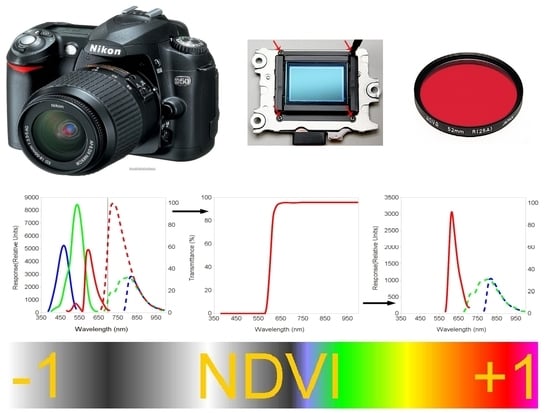
2.1. Transformation of the Nikon D50 in a Full-Spectrum Camera
2.2. Filters Used for the Simultaneous Detection of R and NIR Wavelengths
2.3. Covering the Maximum Possible Variability in the Photographs
2.4. Weighting the RGB Channels in Each Light Condition
2.5. Use of RAW Format
2.6. Using Gretag Macbeth Color Checker to Validate R and NIR Wavelengths
2.7. Statistical Analysis of Reflectance-Brightness Relationship for R and NIR Wavelengths
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ritchter, M.; Weiland, U. Applied Urban Ecology: A Global Framework; Blackwell Publishing: West Sussex, UK, 2012. [Google Scholar]
- Rouse, J.W., Jr.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC Type III Final Report; National Aeronautics and Space Administration NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence. A practical guide. J. Exp. Botany 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Silleos, N.G.; Alexandridis, T.K.; Gitas, I.Z.; Perakis, K. Vegetation indices: Advances made in biomass estimation and vegetation monitoring in the last 30 years. Geocarto Int. 2006, 21, 21–28. [Google Scholar] [CrossRef]
- Rocha, A.V.; Shaver, G.R. Advantages of a two band EVI calculated from solar and photosynthetically active radiation fluxes. Agric. For. Meteorol. 2009, 149, 1560–1563. [Google Scholar] [CrossRef]
- Matsushita, B.; Yang, W.; Chen, J.; Onda, Y.; Qiu, G. Sensitivity of the enhanced vegetation index (EVI) and normalized difference vegetation index (NDVI) to topographic effects: A case study in high-density cypress forest. Sensors 2007, 7, 2636–2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Q.; Cieszewski, C.J.; Madden, M.; Borders, B. A linear mixed-effects model of biomass and volume of trees using Landsat ETM+ images. For. Ecol. Manag. 2007, 244, 93–101. [Google Scholar] [CrossRef]
- Flowers, M.; Weisz, R.; Heiniger, R. Quantitative approaches for using color infrared photography for assessing in-season nitrogen status in winter wheat. Agron. J. 2003, 95, 1189–1200. [Google Scholar] [CrossRef] [Green Version]
- Li, R.H.; Li, X.B.; Li, G.Q.; Wen, W.Y. Simulation of soil nitrogen storage of the typical steppe with the DNDC model: A case study in Inner Mongolia, China. Ecol. Indic. 2014, 41, 155–164. [Google Scholar] [CrossRef]
- Szilagyi, J.; Parlange, M.B. Defining watershed-scale evaporation using a normalized difference vegetation index. J. Am. Water Resour. Assoc. 1999, 35, 1245–1255. [Google Scholar] [CrossRef]
- Martínez, B.; Gilabert, M.A. Vegetation dynamics from NDVI time series analysis using the wavelet transform. Remote Sens. Environ. 2009, 113, 1823–1842. [Google Scholar] [CrossRef]
- He, Y.; Bo, Y.; De Jong, R.; Li, A.; Zhu, Y.; Cheng, J. Comparison of vegetation phenological metrics extracted from GIMMS NDVIg and MERIS MTCI data sets over China. Int. J. Remote Sens. 2015, 36, 300–317. [Google Scholar] [CrossRef]
- Lloret, F.; Lobo, A.; Estevan, H.; Maisongrande, P.; Vayreda, J.; Terradas, J. Woody plant richness and NDVI response to drought events in Catalonian (Northeastern Spain) forests. Ecology 2007, 88, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Lewis, M.M.; Ostendorf, B. An image-based diversity index for assessing land degradation in an arid environment in South Australia. J. Arid Environ. 2008, 72, 1282–1293. [Google Scholar] [CrossRef]
- Maselli, F.; Romanelli, E.; Bottai, L.; Zipoli, G. Use of NOAA-AVHRR NDVI images for the estimation of dynamic fire risk in Mediterranean areas. Remote Sens. Environ. 2003, 86, 187–197. [Google Scholar] [CrossRef]
- Bacaro, G.; Santi, E.; Rocchini, D.; Pezzo, F.; Puglisi, L.; Chiarucci, A. Geostatistical modelling of regional bird species richness: Exploring environmental proxies for conservation purpose. Biodivers. Conserv. 2011, 20, 1677–1694. [Google Scholar] [CrossRef]
- Murphy, R.J.; Underwood, A.J. Novel use of digital colour-infrared imagery to test hypotheses about grazing by intertidal herbivorous gastropods. J. Exp. Mar. Biol. Ecol. 2006, 330, 437–447. [Google Scholar] [CrossRef]
- Oesterheld, M.; DiBella, C.M.; Kerdiles, H. Relation between NOAA-AVHRR Satellite Data and Stocking Rate of Rangelands. Ecol. Appl. 1998, 8, 207–212. [Google Scholar] [CrossRef]
- Thanapura, P.; Helder, D.L.; Burckhard, S.; Warmath, E.; O’Neill, M.; Galster, D. Mapping urban land cover using Quickbird NDVI and GIS spatial modelling for runoff coefficient determination. Photogramm. Eng. Remote Sens. 2007, 73, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Rabatel, G.; Gorretta, N.; Labbé, S. Getting simultaneous red and near-infrared band data from a single digital camera for plant monitoring applications: Theoretical and practical study. Biosyst. Eng. 2014, 117, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, G.L.; Sullivan, D.G.; Perry, C.D.; Hook, J.E.; Bednarz, C.W. Preparation of a low-cost digital camera system for remote sensing. Appl. Eng. Agric. 2008, 24, 885–896. [Google Scholar] [CrossRef]
- Verhoeven, G.J.; Smet, P.F.; Poelman, D.; Vermeulen, F. Spectral characterization of a digital still camera’s NIR modification to enhance archaeological observation. IEEE Trans. Geosci. Remote Sens. 2009, 47, 3456–3468. [Google Scholar] [CrossRef]
- Weekley, J.G. Multispectral Imaging Techniques for Monitoring Vegetative Growth and Health. Master’s Thesis, Virginia Polytechnic Institute, Blacksburg, VA, USA, 2007. [Google Scholar]
- Yang, C.; Everitt, J.H.; Davis, M.R. A CCD camera-based hyperspectral imaging system for stationary and airborne applications. Geocarto Int. 2003, 18, 71–80. [Google Scholar] [CrossRef]
- Verhoeven, G.J. Becoming a NIR-sensitive aerial archaeologist. In Remote Sensing for Agriculture, Ecosystems, and Hydrology IX; Neale, C., Owe, M., D’Urso, G., Eds.; SPIE: Bellingham, WA, USA; Florence, Italy, 2007; pp. 333–345. [Google Scholar]
- Verhoeven, G.J. Near-infrared aerial crop mark archaeology: From its historical use to current digital implementations. J. Archaeol. Method Theory 2012, 19, 132–160. [Google Scholar] [CrossRef]
- Homolová, L.; Maenovsky, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaeprnan, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Verdú, F.; Pujol, J.; Capilla, P. Characterization of a digital camera as an absolute tristimulus. J. Imaging Sci. Technol. 2003, 47, 279–374. [Google Scholar]
- Lebourgeois, V.; Bégué, A.; Labbé, S.; Mallavan, B.; Prévot, L.; Roux, B. Can commercial digital cameras be used as multispectral sensors? A crop monitoring test. Sensors 2008, 8, 7300–7322. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Cohn, R. Dcraw; Book on Demand Ltd.: Miami, FL, USA, 2012. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 5 October 2020).
- Jobson, J.D. Applied Multivariate Data Analysis, 4th ed.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Hier.part: Hierarchical Partitioning. Available online: http://cran.R-project.org/package=hier.part (accessed on 21 May 2019).
- Chevan, A.; Sutherland, M. Hierarchical partitioning. Am. Stat. 1991, 45, 90–96. [Google Scholar]
- Boyd, D.S.; Foody, G.M. An overview of recent remote sensing and GIS based research in ecological informatics. Ecol. Inform. 2011, 6, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, Y.; Tan, Z.; Song, Q.; Liang, N.; Yu, L.; Zhao, J. Using digital cameras for comparative phenological monitoring in an evergreen broad-leaved forest and a seasonal rain forest. Ecol. Inform. 2012, 10, 65–72. [Google Scholar] [CrossRef]






| Parameter | Range |
|---|---|
| Number of photographs | 23 |
| Time | 10 a.m.–18 p.m. |
| Focal distance | 27–72 mm |
| Exposure (Ev) | 6.1–14.3 |
| F-stop | 4.5–20.0 |
| Speed | 0.002–0.17 |
| Red reflectance | 0.03–0.93 |
| Red brightness (16 bits) | 681–65,007 |
| Infrared reflectance | 0.03–0.92 |
| Infrared brightness (16 bits) | 724–65,074 |
| Parameters | R Reflectance~R Brightness | NIR1 Reflectance~G Brightness | NIR2 Reflectance~B Brightness |
|---|---|---|---|
| R = a*exp(b*B) | Rf = 2.35 × 10−2 × exp(5.73 × 10−5 × Br) | Rf = 3.07 × 10−2 × exp(5.61 × 10−5 × Br) | Rf = 5.29 × 10−2 × exp(4.81 × 10−5 × Br) |
| Standard error (a/b) | 3.01 × 10−1/6.83 × 10−7 | 3.02 × 10−2/6.75 × 10−7 | 5.13 × 10−2/1.09 × 10−6 |
| t-value (a/b) | −124.79 ***/83.92 *** | −115.36 ***/83.01 *** | −57.34 ***/43.94 *** |
| R2 | 0.953 | 0.952 | 0.847 |
| Parameter | R Brightness | G Brightness | B Brightness |
|---|---|---|---|
| Time | 31.74 ns | 42.32 ns | 59.06 ns |
| Focal length | 44.57 ns | 15.44 ns | 14.55 ns |
| F-stop | 10.13 ns | 21.48 ns | 11.59 ns |
| Speed | 13.56 ns | 20.77 ns | 14.80 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patón, D. Normalized Difference Vegetation Index Determination in Urban Areas by Full-Spectrum Photography. Ecologies 2020, 1, 22-35. https://0-doi-org.brum.beds.ac.uk/10.3390/ecologies1010004
Patón D. Normalized Difference Vegetation Index Determination in Urban Areas by Full-Spectrum Photography. Ecologies. 2020; 1(1):22-35. https://0-doi-org.brum.beds.ac.uk/10.3390/ecologies1010004
Chicago/Turabian StylePatón, Daniel. 2020. "Normalized Difference Vegetation Index Determination in Urban Areas by Full-Spectrum Photography" Ecologies 1, no. 1: 22-35. https://0-doi-org.brum.beds.ac.uk/10.3390/ecologies1010004