Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review
Abstract
:1. Introduction
2. Natural and Modified Cyclodextrins
2.1. Structure, General Characteristic and Properties of Interest
2.2. α-CD
2.3. β-CD
2.4. γ-CD
2.5. Large Ring CDs
2.6. Modified CDs
3. Preparation of Inclusion Complexes
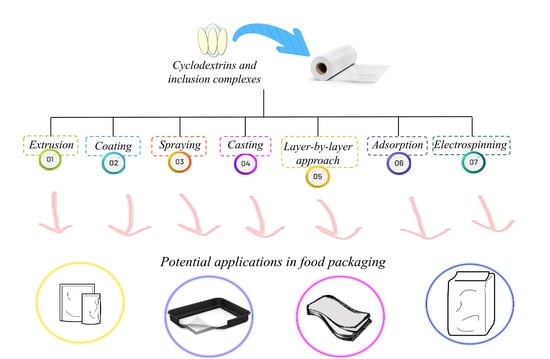
4. Incorporation of CDs/ICs into Polymer Matrices
5. Active Packaging for Food Preservation
5.1. ICs as Active Compounds Carriers
5.2. CDs/ICs as Components of Emitting/Adsorbent Sachets or Pads
6. CDs as Polymer Matrices Components
7. Toxicological and Regulatory Aspects: Food and Food Packaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, E.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, S.; El-Aty, A.M.A.; Hacımüftüoğlu, A.; Yohannes, W.K.; Khan, M.; She, Y. Development of Water-Compatible Molecularly Imprinted Polymers Based on Functionalized β-Cyclodextrin for Controlled Release of Atropine. Polymers 2020, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Mai, N.N.S.; Nakai, R.; Kawano, Y.; Hanawa, T. Enhancing the Solubility of Curcumin Using a Solid Dispersion System with Hydroxypropyl-β-Cyclodextrin Prepared by Grinding, Freeze-Drying, and Common Solvent Evaporation Methods. Pharmacy 2020, 8, 203. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef]
- Hashimoto, H. Present Status of Industrial Application of Cyclodextrins in Japan. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 57–62. [Google Scholar] [CrossRef]
- Novozymes Home Page. Available online: https://www.novozymes.com/en (accessed on 10 September 2021).
- Wacker Chemie AG Home Page. Available online: https://www.wacker.com/cms/en-us/home/home.html (accessed on 10 September 2021).
- Roquette Frères Home Page. Available online: https://www.roquette.com (accessed on 10 September 2021).
- Cyclo Therapeutics, Inc. Home Page. Available online: https://www.cyclodex.com (accessed on 10 September 2021).
- Yeguas, V.; Altarsha, M.; Monard, G.; López, R.; Ruiz-López, M.F. Peptide Binding to β-Cyclodextrins: Structure, Dynamics, Energetics, and Electronic Effects. J. Phys. Chem. A 2011, 115, 11810–11817. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Dias, M.V.; Soares, N.D.F.F.; Borges, S.V.; de Oliveira, I.R.N.; Pires, A.C.D.S.; Medeiros, E.A.A.; Alves, E. Ultrastructural and antimicrobial impacts of allyl isothiocyanate incorporated in cellulose, β-cyclodextrin, and carbon nanotubes nanocomposites. J. Vinyl Addit. Technol. 2021. [Google Scholar] [CrossRef]
- Dermawan, D.; Wathoni, N.; Muchtaridi, M. Host-Guest Interactions of α−Mangostin with (α, β, γ)−Cyclodextrins: Semi-Empirical Quantum Mechanical Methods of PM6 and PM7. J. Young Pharm. 2018, 11, 31–35. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2018, 94, 139–149. [Google Scholar] [CrossRef]
- Kumar, S.; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Enabled Polymer Composites for Packaging. Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as Functional Excipients: Methods to Enhance Complexation Efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Sonnendecker, C.; Melzer, S.; Zimmermann, W. Engineered cyclodextrin glucanotransferases from Bacillus sp. G-825-6 produce large-ring cyclodextrins with high specificity. MicrobiologyOpen 2018, 8, e00757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Complexation of estragole as pure compound and as main component of basil and tarragon essential oils with cyclodextrins. Carbohydr. Polym. 2015, 118, 156–164. [Google Scholar] [CrossRef]
- De Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Buckley, J.D.; Coates, A.M.; Ranald, P.; Howe, C. Fiber Ingredients: Food Applications and Health Benefits, 1st ed.; Cho, S.S., Samuel, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 9–18. ISBN 9780429144776. [Google Scholar]
- U.S. Food and Drug Administration Home Page. Available online: http://www.fda.gov/ (accessed on 10 September 2021).
- Väkeväinen, K.; Rinkinen, N.; Willman, R.-M.; Lappi, J.; Raninen, K.; Kårlund, A.; Mikkonen, S.; Plumed-Ferrer, C.; Kolehmainen, M. Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability. Foods 2021, 10, 792. [Google Scholar] [CrossRef]
- Henck, J.M.; Bis-Souza, C.V.; Pollonio, M.A.; Lorenzo, J.M.; Barretto, A.C.D.S. Alpha-cyclodextrin as a new functional ingredient in low-fat chicken frankfurter. Br. Poult. Sci. 2019, 60, 716–723. [Google Scholar] [CrossRef]
- Bazzano, M.; Barolo, C.; Buscaino, R.; D’Agostino, G.; Ferri, A.; Sangermano, M.; Pisano, R. Controlled Atmosphere in Food Packaging Using Ethylene−α-Cyclodextrin Inclusion Complexes Dispersed in Photocured Acrylic Films. Ind. Eng. Chem. Res. 2016, 55, 579–585. [Google Scholar] [CrossRef]
- Ariyanto, H.D.; Chiba, M.; Oguma, K.; Tatsuki, M.; Yoshii, H. Release behavior of 1-methylcylopropene coated paper-based shellac solution in response to stepwise humidity changes to develop novel functional packaging for fruit. Packag. Technol. Sci. 2019, 32, 523–533. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced Thermal Stability of Eugenol by Cyclodextrin Inclusion Complex Encapsulated in Electrospun Polymeric Nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef]
- Samperio, C.; Boyer, R.; Eigel, W.N.; Holland, K.W.; McKinney, J.S.; O’Keefe, S.F.; Smith, R.; Marcy, J.E. Enhancement of Plant Essential Oils’ Aqueous Solubility and Stability Using Alpha and Beta Cyclodextrin. J. Agric. Food Chem. 2010, 58, 12950–12956. [Google Scholar] [CrossRef]
- Câmara, A.K.F.I.; Paglarini, C.S.; Vidal, V.A.S.; Santos, M.; Pollonio, M.A.R. Advances in Food and Nutrition Research, 1st ed.; da Cruz, A.G., Prudencio, E.S., Esmerino, E.A., da Silva, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 223–265. ISBN 9780128202180. [Google Scholar]
- Pan, J.; Ai, F.; Shao, P.; Chen, H.; Gao, H. Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-β-cyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Wang, J.; Yu, B.; Guo, L.; Dong, D. Mechanical and antimicrobial properties of high amylose corn starch/konjac glucomannan composite film enhanced by cinnamaldehyde/β-cyclodextrin complex. Ind. Crop. Prod. 2021, 170, 113781. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating β-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef]
- Chen, Z.; Zong, L.; Chen, C.; Xie, J. Development and characterization of PVA-Starch active films incorporated with β-cyclodextrin inclusion complex embedding lemongrass (Cymbopogon citratus) oil. Food Packag. Shelf Life 2020, 26, 100565. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2018, 90, 360–366. [Google Scholar] [CrossRef]
- Dias, M.V.; Sousa, M.M.; Lara, B.R.B.; de Azevedo, V.M.; Soares, N.D.F.F.; Borges, S.V.; Queiroz, F. Thermal and morphological properties and kinetics of diffusion of antimicrobial films on food and a simulant. Food Packag. Shelf Life 2018, 16, 15–22. [Google Scholar] [CrossRef]
- Voncina, B.; Vivod, V.; Chen, W.-T. Surface modification of PET fibers with the use of β-cyclodextrin. J. Appl. Polym. Sci. 2009, 113, 3891–3895. [Google Scholar] [CrossRef]
- Szente, L. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Freudenberg, K.; Jacobi, R. Über Schardingers Dextrine aus Stärke. Eur. J. Org. Chem. 1935, 518, 102–108. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous solubility of kinase inhibitors: I the effect of hydrophilic polymers on their γ-cyclodextrin solubilization. J. Drug Deliv. Sci. Technol. 2019, 55, 101462. [Google Scholar] [CrossRef]
- Jansook, P.; Pichayakorn, W.; Muankaew, C.; Loftsson, T. Cyclodextrin–poloxamer aggregates as nanocarriers in eye drop formulations: Dexamethasone and amphotericin B. Drug Dev. Ind. Pharm. 2016, 42, 1446–1454. [Google Scholar] [CrossRef]
- Bastianini, M.; Sisani, M.; Petracci, A. Ascorbyl Tetraisopalmitate Inclusion into Υ-Cyclodextrin and Mesoporous SBA-15: Preparation, Characterization and In Vitro Release Study. Cosmetics 2017, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhu, D.-D.; Ji, G.-J.; Yuan, S.; Qian, J.-F.; He, M.-Y.; Chen, Q.; Zhang, Z.-H. Efficient adsorption separation of xylene isomers using a facilely fabricated cyclodextrin-based metal-organic framework. J. Chem. Technol. Biotechnol. 2018, 93, 2898–2905. [Google Scholar] [CrossRef]
- Savarino, P.; Viscardi, G.; Quagliotto, P.; Montoneri, E.; Barni, E. Reactivity and effects of cyclodextrins in textile dyeing. Dye. Pigment. 1999, 42, 143–147. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, D.; Ren, J.-J.; Dong, R.; Wu, H. Screening of the Repellent Activity of 12 Essential Oils Against Adult German Cockroach (Dictyoptera: Blattellidae): Preparation of a Sustained Release Repellent Agent of Binary Oil-γ-CD and its Repellency in a Small Container. J. Econ. Èntomol. 2020, 113, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhuang, H.; Ran, Y. The Application of Cyclodextrin Glycosyltransferase in Biological Science. J. Bioequivalence Bioavailab. 2011, 3, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Assaf, K.I.; Gabel, D.; Zimmermann, W.; Nau, W.M. High-affinity host–guest chemistry of large-ring cyclodextrins. Org. Biomol. Chem. 2016, 14, 7702–7706. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Ji, M.; Sun, Y.; Zhao, J.; Zhang, H.; Wang, Z. Preparation and characterization of baicalein/hydroxypropyl-β-cyclodextrin inclusion complex for enhancement of solubility, antioxidant activity and antibacterial activity using supercritical antisolvent technology. J. Incl. Phenom. Macrocycl. Chem. 2019, 96, 285–295. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef]
- Mavridis, I.M.; Yannakopoulou, K. Anionic cyclodextrins as versatile hosts for pharmaceutical nanotechnology: Synthesis, drug delivery, enantioselectivity, contrast agents for MRI. Int. J. Pharm. 2015, 492, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sun, H.; Bano, S.; Zhao, Y.; Xu, X.; Tang, J. Preparation, characterization, and in vitro anti-inflammatory evaluation of novel water soluble kamebakaurin/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2017, 1130, 319–326. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ji, M.; Wang, J.; Cheng, C.; Chen, L.; Wen, C.; Zhang, Q. Physicochemical, Antioxidant, In Vitro Release, and Heat Sealing Properties of Fish Gelatin Films Incorporated with β-Cyclodextrin/Curcumin Complexes for Apple Juice Preservation. Food Bioprocess Technol. 2017, 11, 447–461. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Xu, Y.; Huang, H.; Zhao, H.; Wang, J.; Wang, S. Synthesis and characterization of antibacterial polylactic acid film incorporated with cinnamaldehyde inclusions for fruit packaging. Int. J. Biol. Macromol. 2020, 164, 4547–4555. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast-dissolving drug delivery system. Int. J. Pharm. 2020, 584, 119395. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.; Zhou, Y.; Bei, W.; Li, Y.; Yuan, Q.; Liang, H. Characterization of glabridin/hydroxypropyl-β-cyclodextrin inclusion complex with robust solubility and enhanced bioactivity. Carbohydr. Polym. 2017, 159, 152–160. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Kilic, M.E.; Durgun, E.; Uyar, T. Molecular Encapsulation of Cinnamaldehyde within Cyclodextrin Inclusion Complex Electrospun Nanofibers: Fast-Dissolution, Enhanced Water Solubility, High Temperature Stability, and Antibacterial Activity of Cinnamaldehyde. J. Agric. Food Chem. 2019, 67, 11066–11076. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Fabrication of Electrospun Eugenol/Cyclodextrin Inclusion Complex Nanofibrous Webs for Enhanced Antioxidant Property, Water Solubility, and High Temperature Stability. J. Agric. Food Chem. 2018, 66, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Fateminasab, F.; Bordbar, A.; Shityakov, S.; Saboury, A. Molecular insights into inclusion complex formation between β- and γ-cyclodextrins and rosmarinic acid. J. Mol. Liq. 2020, 314, 113802. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Uyar, T. Antioxidant electrospun zein nanofibrous web encapsulating quercetin/cyclodextrin inclusion complex. J. Mater. Sci. 2017, 53, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.A.; Yoshii, H. Release behavior of allyl sulfide from cyclodextrin inclusion complex of allyl sulfide under different storage conditions. Biosci. Biotechnol. Biochem. 2018, 82, 848–855. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Sapkal, N.P.; Kilor, V.; Bhursari, K.P.; Daud, A.S. Evaluation of some Methods for Preparing Gliclazide-β-Cyclodextrin Inclusion Complexes. Trop. J. Pharm. Res. 2007, 6, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lopez, K.J.; Enescu, D.; Torres-Giner, S.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.; Fuciños, P.; Lagaron, J.M. De-velopment of electrospun active films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by the incorporation of cyclodextrin inclusion complexes containing oregano essential oil. Food Hydrocolloids 2020, 108, 106013. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Jiang, Z.-T.; Li, R. Complexation of allyl isothiocyanate with β-cyclodextrin and its derivatives and molecular microcapsule of allyl isothiocyanate in β-cyclodextrin. Eur. Food Res. Technol. 2006, 225, 407–413. [Google Scholar] [CrossRef]
- Han, P.; Zhong, Y.; An, N.; Lu, S.; Wang, Q.; Dong, J. Preparation, characterization, and molecular modeling of sesamol/β-cyclodextrin derivatives inclusion complexes. J. Mol. Liq. 2021, 339, 116790. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. Formation of Rutin–β-Cyclodextrin Inclusion Complexes by Supercritical Antisolvent Precipitation. Polymers 2021, 13, 246. [Google Scholar] [CrossRef]
- Meo, P.L.; D’Anna, F.; Riela, S.; Gruttadauria, M.; Noto, R. Spectrophotometric study on the thermodynamics of binding of α- and β-cyclodextrin towards some p-nitrobenzene derivatives. Org. Biomol. Chem. 2003, 1, 1584–1590. [Google Scholar] [CrossRef] [Green Version]
- Hădărugă, N.G.; Bandur, G.N.; Hădărugă, D.I. Cyclodextrin Fundamentals, Reactivity and Analysis, 1st ed.; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 155–221. ISBN 9783319761596. [Google Scholar]
- Biedermann, F.; Nau, W.; Schneider, H.-J. The Hydrophobic Effect Revisited-Studies with Supramolecular Complexes Imply High-Energy Water as a Noncovalent Driving Force. Angew. Chem. Int. Ed. 2014, 53, 11158–11171. [Google Scholar] [CrossRef] [PubMed]
- Cheriet, M.; Djemil, R.; Khellaf, A.; Khatmi, D. Dopamine Family Complexes with β-Cyclodextrin: Molecular Docking Studies. Polycycl. Aromat. Compd. 2021, 1–10. [Google Scholar] [CrossRef]
- Charumanee, S.; Titwan, A.; Sirithunyalug, J.; Weiss-Greiler, P.; Wolschann, P.; Viernstein, H.; Okonogi, S. Thermodynamics of the encapsulation by cyclodextrins. J. Chem. Technol. Biotechnol. 2006, 81, 523–529. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Shin, J.; Kathuria, A.; Lee, Y.S. Effect of hydrophilic and hydrophobic cyclodextrins on the release of encapsulated allyl isothiocyanate (AITC) and their potential application for plastic film extrusion. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Shlar, I.; Droby, S.; Rodov, V. Antimicrobial coatings on polyethylene terephthalate based on curcumin/cyclodextrin complex embedded in a multilayer polyelectrolyte architecture. Colloids Surfaces B Biointerfaces 2018, 164, 379–387. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, Y.T. Improved thermal stability of polylactic acid (PLA) composite film via PLA–β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K.; Liu, F.-J.; Lou, W.-Y.; Li, N.; Zong, M.-H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/ β -cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2015, 196, 996–1004. [Google Scholar] [CrossRef]
- Barba, C.; Eguinoa, A.; Maté, J.I. Preparation and characterization of β-cyclodextrin inclusion complexes as a tool of a controlled antimicrobial release in whey protein edible films. LWT 2015, 64, 1362–1369. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Zhou, W. The preparation, characterization, anti-ultraviolet and antimicrobial activity of gelatin film incorporated with berberine-HP-β-CD. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 586, 124273. [Google Scholar] [CrossRef]
- Mallardo, S.; De Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; Di Lorenzo, M.L. Poly(butylene succinate)-based composites containing β-cyclodextrin/d-limonene inclusion complex. Eur. Polym. J. 2016, 79, 82–96. [Google Scholar] [CrossRef]
- Jia, X.; Yang, N.; Qi, X.; Chen, L.; Zhao, Y. Adsorptive removal of cholesterol by biodegradable zein-graft-β-cyclodextrin film. Int. J. Biol. Macromol. 2020, 155, 293–304. [Google Scholar] [CrossRef]
- Olmo, J.A.-D.; Álvarez, L.P.; Hernáez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)- β-cyclodextrin onto polylactic acid (PLLA). Food Hydrocoll. 2018, 88, 228–236. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Gallur, M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Immobilization of β-cyclodextrin in ethylene-vinyl alcohol copolymer for active food packaging applications. J. Membr. Sci. 2010, 353, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Lizundia, E.; Gómez-Galván, F.; Pérez-Álvarez, L.; León, L.; Vilas, J. Poly(L-lactide)/branched β-cyclodextrin blends: Thermal, morphological and mechanical properties. Carbohydr. Polym. 2016, 144, 25–32. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 733. ISBN 9781439862414. [Google Scholar]
- Wrona, M.; Silva, F.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M. Design of new natural antioxidant active packaging: Screening flowsheet from pure essential oils and vegetable oils to ex vivo testing in meat samples. Food Control. 2020, 120, 107536. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EC) Nº. 450/2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food. 2009. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:135:0003:0011:EN:PDF (accessed on 16 September 2021).
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2018, 3, 77–96. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2017, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Restuccia, D.; Salomone, R.; Spizzirri, U.G.; Saija, G.; Ioppolo, G.; Parisi, O.I.; Picci, N. Industrial applications: Regulatory issues and life cycle assessment of food packaging, 1st ed.; Academic Press: London, UK, 2016; pp. 215–227. ISBN 9780128007235. [Google Scholar]
- Ayala-Zavala, J.F.; González-Aguilar, G.A. Optimizing the Use of Garlic Oil as Antimicrobial Agent on Fresh-Cut Tomato through a Controlled Release System. J. Food Sci. 2010, 75, M398–M405. [Google Scholar] [CrossRef] [PubMed]
- Mitsubishi Chemical Corporation Home Page. Available online: https://www.mfc.co.jp/wasaouro/eng/about/index.html (accessed on 22 September 2021).
- Marques, C.S.; Grillo, R.P.; Bravim, D.G.; Pereira, P.V.; Villanova, J.C.O.; Pinheiro, P.F.; Carneiro, J.C.S.; Bernardes, P.C. Preservation of ready-to-eat salad: A study with combination of sanitizers, ultrasound, and essential oil-containing β-cyclodextrin inclusion complex. LWT 2019, 115, 108433. [Google Scholar] [CrossRef]
- Novipax Home Page. Available online: http://www.novipax.com/markets/fresh-produce/ (accessed on 22 September 2021).
- Silva, F.; Domingues, F.C.; Nerín, C. Control microbial growth on fresh chicken meat using pinosylvin inclusion complexes based packaging absorbent pads. LWT 2018, 89, 148–154. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldaña, M.D.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Woltman, G.R.; Liu, Y.; Quincy, R.B., III. Water Permeable Porous Layer Materials Treated with Surfactant-Modified Cyclodextrins. U.S. Patent 6,433,243 B1, 13 August 2002. [Google Scholar]
- Carlucci, G.; Guarracino, M.; di Cintio, A. Cyclodextrin-Containing Odour Control Material for Absorbent Articles. EP 0894502A1, 3 February 1999. [Google Scholar]
- Chiu, S.-H.; Chung, T.-W.; Giridhar, R.; Wu, W.-T. Immobilization of β-cyclodextrin in chitosan beads for separation of cholesterol from egg yolk. Food Res. Int. 2004, 37, 217–223. [Google Scholar] [CrossRef]
- Dias, H.; Berbicz, F.; Pedrochi, F.; Baesso, M.; Matioli, G. Butter cholesterol removal using different complexation methods with beta-cyclodextrin, and the contribution of photoacoustic spectroscopy to the evaluation of the complex. Food Res. Int. 2010, 43, 1104–1110. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Domenico, A.D.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Remy, U.G.; Leblanc, J.C.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, 4628. [Google Scholar] [CrossRef]
- European Union. Commission directive 2003/95/EC of 27 October 2003 amending Directive 96/77/EC laying down specific purity criteria on food additives other than colours and sweeteners. Off. J. Eur. Union 2003, L283/77, 71–77. [Google Scholar]
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2015, 56, 1981–2004. [Google Scholar] [CrossRef]




| Property | α-Cyclodextrin | β-Cyclodextrin | γ-Cyclodextrin |
|---|---|---|---|
| Commercial name on the Market | Cavamax® W6 [8], Trappsol® Native Alpha [10] | Cavamax® W7 [8], Kleptose® [9], Trappsol® Native Beta [10] | Cavamax® W8 [8], Trappsol® Native Gamma [10] |
| Functions on Markets products | Solubilizer, stabilizer, delivery of drugs [8,10] | Solubilizer, stabilizer, delivery of drugs and taste-masking agent [9,10] | Solubilizer, stabilizer, stabilization enhancer, delivery of drugs [8,10] |
| Number of glucose subunits | 6 | 7 | 8 |
| Molar mass (g·mol−1) | 972 | 1135 | 1297 |
| External size (nm) | 1.4–1.5 [19] | 1.5–1.6 [19] | 1.7–1.8 [19] |
| Internal diameter (nm) | 0.47–0.52 [21] | 0.60–0.80 [21] | 0.75–1.00 [21] |
| Water solubility at 25 °C(mg·mL−1) | 145 [21] | 18.5 [21] | 232 [21] |
| Solubility in organic solvents | Insoluble in chloroform, isopropanol, acetone, ethanol, glycerin, methanol, and ethyl ether. Soluble in propylene glycol (10 mg·mL−1), dimethyl sulfoxide (20 mg·mL−1), and dimethylformamide (540 mg·mL−1) [21] | Insoluble in chloroform, isopropanol, acetone, ethanol, methanol, and ethyl ether. Soluble in dimethyl sulfoxide (350 mg·mL−1), ethylene glycol (210 mg·mL−1), dimethylformamide (320 mg·mL−1), and glycerin (43 mg·mL−1) [21] | Insoluble in chloroform and ethyl ether. Soluble (>1 mg·mL−1) in isopropanol, acetone, ethanol, and methanol [21] |
| CD | Guest Molecule | Main Effects of Complexation | Reference |
|---|---|---|---|
| HP-γ-CD | Ferulic acid | Accelerating dissolution of oral-introduced medicinal products | [60] |
| HP-β-CD | Glabridin | Increased water solubility and bioactivity | [61] |
| β-CD | Basil and Pimenta dioica EOs | Increased the EOs thermal stability | [38] |
| α-CD | Moringin | Increased water solubility and verification of anti-inflammatory effect | [62] |
| HP-γ- and HP-β-CD | Cinnamaldehyde | Increased thermal stability, increased solubility and dissolution in water, maintenance of antimicrobial activity against Escherichia coli | [63] |
| HP-γ- and HP-β-CD | Curcumin | Increased solubility in water, which promoted higher antioxidant activity | [64] |
| HP-γ-, HP-β-, and methyl-β-CD (M-β-CD) | Thymol | Reduced volatility, increased water solubility, rapid disintegration in water, increased thermal stability | [65] |
| α-,β-, HP-β-, γ-CD, randomly methylated CD (RAMEB), and low methylated CD | Estragole | Higher photostability and antioxidant activity, controlled release of the guest molecule | [20] |
| β- and γ-CD | Rosmarinic acid | Increased stability, solubility, bioavailability, antioxidant and anti-inflammatory activities | [66] |
| γ-CD | Quercetin | Increased water solubility of the guest molecule stability | [67] |
| α-CD | Allyl sulfide | Controlled release of the guest molecule | [68] |
| HP-β-CD, HP-γ-CD and M-β-CD | Linalool | Increased thermal stability, rapid dissolution and controlled release of the active compound, antimicrobial activity against Gram-positive and Gram-negative bacteria | [69] |
| β-CD | Garlic EO | Thermal protection | [14] |
| CD | Guest Molecule | Packaging Polymer | Purpose of CD/IC in Packaging | Reference |
|---|---|---|---|---|
| As inclusion complex | ||||
| HP-β-CD | Gallic acid | Polylactic acid (PLA) nanofibers | Promote the controlled release of the active compound | [84] |
| β-CD | PLA | PLA | Increase the thermal stability of PLA films | [85] |
| β-CD | Cinnamon EO | PLA nanofibers | Manufacture an antimicrobial film with increased thermal stability of the bioactive molecule | [86] |
| α- and γ-CD | Oregano EO | Poly (3-hydroxybutyrate-co-3-hydroxy valerate) (PHBV) | Increase thermal stability of the bioactive compound for film making at high temperatures | [72] |
| β-CD | Cinnamaldehyde | PLA | Improve mechanical and barrier properties, in addition to promote the controlled release of the bioactive molecule (active packaging) | [59] |
| β-CD | Eugenol and carvacrol | Whey protein | Promote the controlled release of antimicrobial bioactive compounds in edible films | [87] |
| Carboxymethyl-β-CD | Curcumin | Polyethylene-terephthalate (PET) | Develop an antimicrobial film with controlled release | [83] |
| HP-β-CD | Berberine | Gelatin | Preparation of antibacterial films with anti-ultraviolet properties (increased solubility and thermal stability of the active compound) | [88] |
| β-CD | D-limonene | Poly (butylene-succinate) (PBS) | Thermal stabilization of the active compound and improvement of the thermal properties of PBS films | [89] |
| β-CD | Allyl isothyocianate | Cellulose acetate | Promote the controlled release of the active agent | [40] |
| As a polymer blend component | ||||
| β-CD | Cinnamon EO | Polyvinyl alcohol (PVA) (nanofibers) | Control the EO odor and ensure a more controlled release to obtain antimicrobial active packaging | [33] |
| β-CD | - | Zein | Cholesterol absorption | [90] |
| β-CD | Allyl isothyocianate | Low-density polyethylene (LDPE) | Promote the controlled release of the active agent | [82] |
| (2-carboxyethyl)-β-CD | - | Chitosan and PLLA (loaded with ZnO nanoparticles–multilayer) | Improve PLLA functionality as packaging material | [91] |
| β-CD | Cinnamon and oregano EOs | Chitosan and PVA (multilayer) | Improve the release of EOs and prepare films with antifungal activity | [92] |
| β-CD | - | Ethylene-vinyl alcohol (EVOH) | Promote changes in the morphological, thermal and barrier properties of EVOH | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, T.R.; Marques, C.S.; Soares, N.F.F. Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review. Polysaccharides 2021, 2, 825-842. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2040050
Arruda TR, Marques CS, Soares NFF. Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review. Polysaccharides. 2021; 2(4):825-842. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2040050
Chicago/Turabian StyleArruda, Tarsila R., Clara S. Marques, and Nilda F. F. Soares. 2021. "Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review" Polysaccharides 2, no. 4: 825-842. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2040050