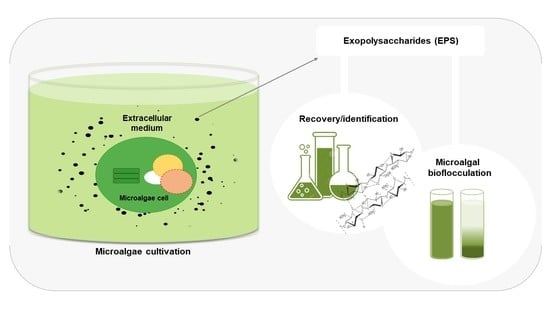
Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants
Abstract
:1. Introduction
2. Potentiality of Microalgal Polysaccharides in Bioflocculation
3. Recent Advances in Harvesting Algae and Pretreatments for the Extraction of Cell-Bound EPS
4. Techniques for Recovery/Identification of Microalgae Polysaccharides
5. Application of Microalgal Bioflocculants in Microalgae Harvesting
5.1. Bioflocculation
5.2. Autoflocculation
6. Other Applications of Microalgal EPS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Moraes, L.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Spirulina sp. LEB 18 cultivation in seawater and reduced nutrients: Bioprocess strategy for increasing carbohydrates in biomass. Bioresour. Technol. 2020, 316, 123883. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, P.Q.M.; Moraes, L.; Silva, T.N.M.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Innovative application of brackish groundwater without the addition of nutrients in the cultivation of Spirulina and Chlorella for carbohydrate and lipid production. Bioresour. Technol. 2022, 345, 126543. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Cuevas-Castillo, G.A.; Navarro-Pineda, F.S.; Baz Rodríguez, S.A.; Sacramento Rivero, J.C. Advances on the processing of microalgal biomass for energy-driven biorefineries. Renew. Sustain. Energy Rev. 2020, 125, 109606. [Google Scholar] [CrossRef]
- Salim, S.; Kosterink, N.R.; Tchetkoua Wacka, N.D.; Vermuë, M.H.; Wijffels, R.H. Mechanism behind autoflocculation of unicellular green microalgae Ettlia texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef]
- Guo, S.L.; Zhao, X.Q.; Wan, C.; Huang, Z.Y.; Yang, Y.L.; Asraful Alam, M.; Ho, S.H.; Bai, F.W.; Chang, J.S. Characterization of flocculating agent from the self-flocculating microalga Scenedesmus obliquus AS-6-1 for efficient biomass harvest. Bioresour. Technol. 2013, 145, 285–289. [Google Scholar] [CrossRef]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of microalgae by bio-flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Wan, C.; Guo, S.L.; Zhao, X.Q.; Huang, Z.Y.; Yang, Y.L.; Chang, J.S.; Bai, F.W. Characterization of the flocculating agent from the spontaneously flocculating microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014, 118, 29–33. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Cheng, S.; Zhang, W.; Zhang, X. Enhanced Microalgal Harvesting Using Microalgae-Derived Extracellular Polymeric Substance as Flocculation Aid. ACS Sustain. Chem. Eng. 2020, 8, 4069–4075. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Uemura, Y.; Thanh, N.T. Flocculation and mechanism of self-flocculating lipid producer microalga Scenedesmus quadricauda for biomass harvesting. Biomass Bioenergy 2016, 93, 38–42. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Boonchai, R.; Kaewsuk, J.; Seo, G. Effect of nutrient starvation on nutrient uptake and extracellular polymeric substance for microalgae cultivation and separation. Desalin. Water Treat. 2014, 55, 360–367. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulfated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-κB and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef]
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2009, 26, 833–840. [Google Scholar] [CrossRef]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Majdoub, H.; Mansour, M.B.; Chaubet, F.; Roudesli, M.S.; Maaroufi, R.M. Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim. Biophys. Acta 2009, 1790, 1377–1381. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Wang, H.; Qi, B.; Jiang, X.; Jiang, Y.; Yang, H.; Xiao, Y.; Jiang, N.; Deng, L.; Wang, W. Microalgal interstrains differences in algal-bacterial biofloc formation during liquid digestate treatment. Bioresour. Technol. 2019, 289, 121741. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Kumar, D.; Kvíderová, J.; Kaštánek, P.; Lukavský, J. The green alga Dictyosphaerium chlorelloides biomass and polysaccharides production determined using cultivation in crossed gradients of temperature and light. Eng. Life Sci. 2017, 17, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Xu, X.; Fujibayashi, M.; Niu, Q.; Tanaka, N.; Nishimura, O. Response of microalgae to elevated CO2 and temperature: Impact of climate change on freshwater ecosystems. Environ. Sci. Pollut. Res. Int. 2016, 23, 19847–19860. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, S.; Zhang, J.; Nguyen, B.T.; Zhang, Z. Insight into the influences of pH value on Pb(II) removal by the biopolymer extracted from activated sludge. Chem. Eng. J. 2017, 308, 1098–1104. [Google Scholar] [CrossRef]
- Raungsomboon, S.; Chidthaisong, A.; Bunnag, B.; Inthorn, D.; Harvey, N.W. Production, composition and Pb2+ adsorption characteristics of capsular polysaccharides extracted from a cyanobacterium Gloeocapsa gelatinosa. Water Res. 2006, 40, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Patwal, T.; Baranwal, M. Scenedesmus acutus extracellular polysaccharides produced under increased concentration of sulphur and phosphorus exhibited enhanced proliferation of peripheral blood mononuclear cells. 3 Biotech 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar. Drugs 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, Z.; Wang, J.; Liu, A.; Li, Z.; Yu, R.; Wu, X.; Liu, Y.; Li, J.; Zeng, W. Characterization of extracellular polysaccharide/protein contents during the adsorption of Cd(II) by Synechocystis sp. PCC6803. Environ. Sci. Pollut. Res. Int. 2018, 25, 20713–20722. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Noguera, D.R.; Zhao, N.; Song, Y.; Ding, J.; Zhao, Q.; Cui, F. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z. Cadmium(II) sequestration characteristics by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresour. Technol. 2010, 101, 9742–9748. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef]
- Koçer, A.T.; İnan, B.; Kaptan Usul, S.; Özçimen, D.; Yılmaz, M.T.; Işıldak, İ. Exopolysaccharides from microalgae: Production, characterization, optimization and techno-economic assessment. Braz. J. Microbiol. 2021, 52, 1779–1790. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M. Influence of bioflocculation parameters on harvesting Chlorella salina and its optimization using response surface methodology. J. Environ. Chem. Eng. 2013, 4, 1051–1056. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, H.P.; Fu, Q.; Johir, M.A.H.; Ibrahim, I.; Mofijur, M.; Labeeuw, L.; Pernice, M.; Ralph, P.J.; Nghiem, L.D. Synthesis and evaluation of cationic polyacrylamide and polyacrylate flocculants for harvesting freshwater and marine microalgae. Chem. Eng. J. 2022, 433, 133623. [Google Scholar] [CrossRef]
- Choi, O.K.; Hendren, Z.; Kim, G.D.; Dong, D.; Lee, J.W. Influence of activated sludge derived-extracellular polymeric substance (ASD-EPS) as bio-flocculation of microalgae for biofuel recovery. Algal Res. 2020, 45, 101736. [Google Scholar] [CrossRef]
- Pandey, A.; Pathak, V.V.; Kothari, R.; Black, P.N.; Tyagi, V.V. Experimental studies on zeta potential of flocculants for harvesting of algae. J. Environ. Manag. 2019, 231, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]
- Ghazvini, M.; Kavosi, M.; Sharma, R.; Kim, M. A review on mechanical-based microalgae harvesting methods for biofuel production. Biomass Bioenergy 2022, 158, 106348. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Barajas-Fernández, J.; Olán-Acosta, M.d.l.A.; Petriz-Prieto, M.A.; Guzmán-López, A.; Bravo-Sánchez, M.G. Techno-Economic Study of CO2 Capture of a Thermoelectric Plant Using Microalgae (Chlorella vulgaris) for Production of Feedstock for Bioenergy. Energies 2020, 13, 413. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Zou, X.; Chang, W.; Qu, Y.; Li, Y. Microalgae harvesting technique using ballasted flotation: A review. Set. Purif. Tecnol. 2021, 276, 119439. [Google Scholar] [CrossRef]
- Parmentier, D.; Manhaeghe, D.; Baccini, L.; Meirhaeghe, R.V.; Rousseau, D.P.L.; Hulle, S.V. A new reactor design for harvesting algae through electrocoagulation-flotation in a continuous mode. Algal Res. 2020, 47, 101828. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent progress in flocculation, dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, T.; Li, S.; Nugroho, Y.K.; Li, B.; Cao, J.; Show, P.-L.; Hiltunen, E. Effects of operating parameters on algae Chlorella vulgaris biomass harvesting and lipid extraction using metal sulfates as flocculants. Biomass Bioenergy 2020, 132, 105433. [Google Scholar] [CrossRef]
- Mayers, J.J.; Landels, A.R.; Allen, M.J.; Albers, E. An energy and resource efficient alkaline flocculation and sedimentation process for harvesting of Chromochloris zofingiensis biomass. Bioresour. Technol. Rep. 2020, 9, 100358. [Google Scholar] [CrossRef]
- Smith, B.T.; Davis, R.H. Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Res. 2012, 1, 32–39. [Google Scholar] [CrossRef]
- Yang, F.; Xiang, W.; Fan, J.; Wu, H.; Li, T.; Long, L. High pH-induced flocculation of marine Chlorella sp. for biofuel production. J. Appl. Phycol. 2016, 28, 747–756. [Google Scholar] [CrossRef]
- Vandamme, D.; Beuckels, A.; Markou, G.; Foubert, I.; Muylaert, K. Reversible Flocculation of Microalgae using Magnesium Hydroxide. Bioenergy Res. 2015, 8, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Mayers, J.J.; Vaiciulyte, S.; Malmhäll-Bah, E.; Alcaide-Sancho, J.; Ewald, S.; Godhe, A.; Ekendahl, S.; Albers, E. Identifying a marine microalgae with high carbohydrate productivities under stress and potential for efficient flocculation. Algal Res. 2018, 31, 430–442. [Google Scholar] [CrossRef]
- Cancela, Á.; Sánchez, Á.; Álvarez, X.; Jiménez, A.; Ortiz, L.; Valero, E.; Varela, P. Pellets valorization of waste biomass harvested by coagulation of freshwater algae. Bioresour. Technol. 2016, 204, 152–156. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Emmerton, B.; Wang, Q.; Ralph, P.J.; Nghiem, L.D. Factors governing microalgae harvesting efficiency by flocculation using cationic polymers. Bioresour. Technol. 2021, 340, 125669. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, F.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Extraction of extracellular polymeric substances (EPS) from a newly isolated self-flocculating microalga Neocystis mucosa SX with different methods. Algal Res. 2019, 40, 101479. [Google Scholar] [CrossRef]
- Huang, R.; He, Q.; Ma, J.; Ma, C.; Xu, Y.; Song, J.; Sun, L.; Wu, Z.; Huangfu, X. Quantitative assessment of extraction methods for bound extracellular polymeric substances (B-EPSs) produced by Microcystis sp. and Scenedesmus sp. Algal Res. 2021, 56, 102289. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Wang, H.; Huo, H.; Guo, B.; Liu, N.; Zhang, A.; Niu, S. Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. J. Appl. Phycol. 2020, 32, 1569–1579. [Google Scholar] [CrossRef]
- Balti, R.; Le Balc’h, R.; Brodu, N.; Gilbert, M.; Le Gouic, B.; Le Gall, S.; Sinquin, C.; Massé, A. Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem. 2018, 74, 175–184. [Google Scholar] [CrossRef] [Green Version]
- García-Cubero, R.; Wang, W.; Martín, J.; Bermejo, E.; Sijtsma, L.; Togtema, A.; Barbosa, M.J.; Kleinegris, D.M.M. Milking exopolysaccharides from Botryococcus braunii CCALA778 by membrane filtration. Algal Res. 2018, 34, 175–181. [Google Scholar] [CrossRef]
- Zaouk, L.; Massé, A.; Bourseau, P.; Taha, S.; Rabiller-Baudry, M.; Jubeau, S.; Teychené, B.; Pruvost, J.; Jaouen, P. Filterability of exopolysaccharides solutions from the red microalga Porphyridium cruentum by tangential filtration on a polymeric membrane. Environ. Technol. 2020, 41, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Macao, V.; Gardarin, C.; Rihouey, C.; Picton, L.; Michaud, P.; Laroche, C. The red microalga Flintiella sanguinaria as a new exopolysaccharide producer. J. Appl. Phycol. 2018, 30, 2803–2814. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Biological pretreatment of lignocellulosic biomass—Current trends and future perspectives. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. [Google Scholar]
- Lv, J.; Guo, B.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Integration of wastewater treatment and flocculation for harvesting biomass for lipid production by a newly isolated self-flocculating microalga Scenedesmus rubescens SX. J. Clean. Prod. 2019, 240, 118211. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, J.; Lv, M.; Yu, H.; Zhao, H.; Xu, X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere 2015, 121, 26–32. [Google Scholar] [CrossRef]
- De Brouwer, J.F.C.; Wolfstein, K.; Ruddy, G.K.; Jones, T.E.R.; Stal, L.J. Biogenic stabilization of intertidal sediments: The importance of extracellular polymeric substances produced by benthic diatoms. Microb. Ecol. 2005, 49, 501–512. [Google Scholar] [CrossRef]
- Charcosset, C. Membrane processes in biotechnology: An overview. Biotechnol. Adv. 2006, 24, 482–492. [Google Scholar] [CrossRef]
- Mishra, A.; Jha, B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour. Technol. 2009, 100, 3382–3386. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Zhu, L. Self-flocculation as an efficient method to harvest microalgae: A mini-review. Water 2021, 13, 2585. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, L.; Zhang, H.; Li, C.; Zhang, X.; Hu, Q. A novel low cost microalgal harvesting technique with coagulant recovery and recycling. Bioresour. Technol. 2018, 266, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, C.N.; Nwoba, E.G. Bio-based flocculants for sustainable harvesting of microalgae for biofuel production. A review. Renew. Sustain. Energy Rev. 2021, 139, 110690. [Google Scholar] [CrossRef]
- Salim, S.; Vermuë, M.H.; Wijffels, R.H. Ratio between autoflocculating and target microalgae affects the energy-efficient harvesting by bio-flocculation. Bioresour. Technol. 2012, 118, 49–55. [Google Scholar] [CrossRef]
- Wan, C.; Alam, M.A.; Zhao, X.-Q.; Zhang, X.-Y.; Guo, S.-L.; Ho, S.-H.; Chang, J.-S.; Bai, F.-W. Current progress and future prospect of microalgal biomass harvest using various flocculation technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, Z.; Chen, C.; Xu, X. An auto-flocculation strategy for Chlorella vulgaris. Biotechnol. Lett. 2015, 37, 75–80. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae autoflocculation: An alternative to high-energy consuming harvesting methods. J. Appl. Phycol. 2013, 25, 991–999. [Google Scholar] [CrossRef]
- Jesus, C.S.d.; Jesus Assis de, D.; Rodriguez, M.B.; Menezes Filho, J.A.; Costa, J.A.V.; de Souza Ferreira, E.; Druzian, J.I. Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int. J. Biol. Macromol. 2019, 124, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, C.; Zhao, L.; Zhang, Y.; Yang, J.; Song, L.; Yang, S. Bioflocculant produced by Chlamydomonas reinhardtii. J. Appl. Phycol. 2012, 24, 1245–1251. [Google Scholar] [CrossRef]
- Zhao, F.; Xiao, J.; Ding, W.; Cui, N.; Yu, X.; Xu, J.W.; Li, T.; Zhao, P. An effective method for harvesting of microalga: Coculture-induced self-flocculation. J. Taiwan Inst. Chem. Eng. 2019, 100, 117–126. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-based system for removal of emerging pollutants from wastewater: A review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rao, B.; Wu, P.; Liu, Y.; Li, G.; Li, D. Development of artificially induced biological soil crusts in fields and their effects on top soil. Plant Soil 2013, 370, 115–124. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Bhunia, B.; Mondal, A.; Gopikrishna, K.; Indrama, T. System metabolic engineering of exopolysaccharide-producing cyanobacteria in soil rehabilitation by inducing the formation of biological soil crusts: A review. J. Clean. Prod. 2019, 211, 70–82. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.k.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Alvarez, X.; Alves, A.; Ribeiro, M.P.; Lazzari, M.; Coutinho, P.; Otero, A. Biochemical characterization of Nostoc sp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021, 254, 117303. [Google Scholar] [CrossRef]
- Uhliariková, I.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kim, H.J.; Park, Y.I.; Matulová, M.; Lukavský, J.; Hromadková, Z.; Capek, P. Structural characteristics and biological effects of exopolysaccharide produced by cyanobacterium Nostoc sp. Int. J. Biol. Macromol. 2020, 160, 364–371. [Google Scholar] [CrossRef]

| Drying Process | Harvest Method | Responses | |||
|---|---|---|---|---|---|
| Electrical Power (kWh Year−1) * | Unit Production Cost (US $ kg−1) * | Operating Cost (US $ Year−1) * | Biomass Production (kg Year−1) | ||
| Spray drying | Auto-flocculation followed by vacuum filtering | 13,988 | 1.25 | 61,000 | 48,145.6–53,495.11 |
| Auto-flocculation followed by plate press filter | 8738 | 1.19 | 58,000 | ||
| Drum dryer | Auto-flocculation followed by plate press filter | 8297 | 0.85 | 42,000 | 48,145.6–53,495.11 |
| Auto-flocculation followed by vacuum filtering | 13,597 | 0.91 | 45,000 | ||
| Spray drying | Adding an iron salt followed by vacuum filtering | 13,987 | 1.21 | 59,000 | 48,145.6–54,306.51 |
| Adding an iron salt followed by plate press filter | 8737 | 1.15 | 56,000 | ||
| Drum dryer | Adding an iron salt followed by plate press filter | 8296 | 0.81 | 40,000 | 48,145.6–54,306.51 |
| Adding an iron salt followed by vacuum filtering | 13,546 | 0.87 | 43,000 | ||
| Spray drying | Flocculation with chitosan followed by vacuum filtering | 13,988 | 1.26 | 62,000 | 48,145.6–54,306.51 |
| Flocculation with chitosan followed by plate press filter | 8728 | 1.20 | 59,000 | ||
| Drum dryer | Flocculation with chitosan followed by plate press filter | 8297 | 0.86 | 43,000 | 48,145.6–54,306.51 |
| Flocculation with chitosan followed by vacuum filtering | 25,597 | 0.92 | 46,000 | ||
| Microalgae | Recovery Process | Experimental Conditions | Substance Used in Biomass Recovery | Process Yield | Reference |
|---|---|---|---|---|---|
| Chromochloris zofingiensis | Flocculation and alkaline sedimentation | Bold’s Basal Medium, biomass concentration 0.5, 1.0, 1.5 g L−1, 200 L working volume, air sparged (~10 L min−1), centrifugation 12,000× g for 10 min, pH 7.0 | NaOH (4.6 and 8 mM) and MgSO4 (6, 8 and 10 mM) | Sedimentation yield above 90% | [52] |
| Chlorella vulgaris UTEX 395 | Flocculation with naturally available magnesium in brackish water | BG-11 medium, biomass concentration 0.3% v v−1, stirring at 700 rpm for 5 min, pH 9.0 | Mg2+ (0.3 mM) and MgCl2 (9.6 mM) | Sedimentation yield of 100 cm h−1 | [53] |
| Chlorella marinha sp. | Flocculation induced by NaOH | F/2 medium Guillard, 5000× g for 5 min | NaOH (5 and 7 mM) | Flocculation yield of 90% | [54] |
| Chlorella vulgaris | Reversible flocculation | Wright’s Cryptophyte medium, 10 L working volume, centrifugation 20 min of stirrung at 250 rpm, pH 8.5 | Mg (2.5 mM) and NaOH (4 mM) | Maximum flocculation efficiency of 90% | [55] |
| Phaeodactylum tricornutum | Maximum flocculation efficiency of 73% | ||||
| Chlorella salina | Alkaline autoflocculation | Guillard’s F/2 media with artificial seawater media, optical density of 0.1, 11,500× g for 12 min, pH 8.0 | NaOH (4 mM) | Biomass recoveries greater than 95% efficiency | [56] |
| Scenedesmus sp., Kirchneriella sp., and Microcystis aeruginosa | Induced flocculation | Medium mixture, 50 L working volume, 200 rpm for 1 min, pH 7.7 | CaCl2 (20, 60, 120 and 180 mg L−1), NaC6H7O6 (10 and 20 mg L−1) and Tannin (10 and 20 mg L−1). | Maximum flocculation efficiency for Tannin 95.35%, sodium alginate 90.49% and, calcium chloride 84.04% | [57] |
| Chlorella vulgaris | Induced natural flocculation | N8 medium, 1 L working volume, 100 rpm for over 24 h, pH 6.8 | Chitosan (0.25 g L−1) and aluminum sulfate (2.5 g L−1) | Flocculation yield of 90% | [58] |
| Chlorella vulgaris | Induced flocculation | MLA medium, 350 L working volume, 100% CO2 for 1 min d−1, pH 9.0 | Cationic polyacrylamide polymer (2 g L−1) | Flocculation yield of 97% | [59] |
| Techniques | Microalga | Process Conditions | Objective | Responses | Reference |
|---|---|---|---|---|---|
| Membrane filtration | Porphyridium cruentum | Permeate fluxes of 49.8, 68.9 and 81.9 L h−1 m−2 and 4 bar for, respectively, cross-flow velocities of 2.5, 3.3 and 4.2 m s−1; 49.7 L h−1 m−2. | Influence of cross-flow velocities on filtration performances. | EPS concentration at 6.3 to 10.4 times reaching from 1.74–2.26 g L−1 (80% (w w−1) recovery). | [63] |
| Membrane filtration | Botryococcus braunii CCALA778 | Culture flow circulating in 110 cm2 area 0.2 μm microfiltration hollow fiber membrane (GE Healthcare®, CFP-2-E-35MA). | Optimization and efficiency of extraction and recovery, ensuring high efficiency without compromising the viability of the culture. | Increased EPS productivity by 25% (4 g m−2 d−1). Daily EPS extraction rate of 0.36 g m−2 d−1. | [64] |
| Ultrafiltration (polymeric membrane) | Porphyridium cruentum | PES 50 kDa flat membrane in full recirculation mode, with permeate flow transmembrane pressure (TMP) curves (0.10–1.06 kg GlcEq m−3), tangential fluid velocity (0.3–1.2 m s−1), and temperature (20 and 40 °C). | Parametric study of ultrafiltration of EPS solutions in organic membrane. | The concentrated solution of 0.10 kg GlcEq m−3 (moderate fouling, portion of irreversible/reversible fouling was 88 and 12%). | [65] |
| Diafiltration | Flintiella sanguinaria | Vivaflow ultrafiltration system (Sartorius) and 100 kDa NMWCO membranes. | Native EPS extraction. | EPS solution was concentrated (volume reduction factor of 5). | [66] |
| High Pressure Anion Exchange Chromatography (HPAEC) | Flintiella sanguinaria | The quantification of monosaccharides was achieved by injecting different concentrations of monosaccharides and plotting the response area as a function of concentration. | Quantification and identification of native EPS extract. | Identification of galactoxylan, with rhamnose and glucuronic acid, low content of sulfate groups (0.6%), and methylated and acetylated compounds (5.1 and 3.2%, w w−1). | [66] |
| Molecular weight (Mw) | Chlorella zofingiensis | Determination with gel permeation chromatography and refraction detector (RI), and at a flow rate of 0.5 mL min−1 using 0.2 M sodium nitrate as the mobile phase. | Investigation of the physicochemical characteristics of EPS. | EPS yields were 208.4 and 364.3 mg L−1 with average molecular weights of 2.66 × 104 and 1.88 × 104 Da, respectively. | [20] |
| Microalga | Description of the Experimental Set | Chemical Composition and Identification of Bioflocculants | Produced and Applied Concentration of Bioflocculants | Bioflocculant Application | Reference |
|---|---|---|---|---|---|
| Spirulina sp. LEB-18 | Outdoor cultivation, using a raceway (250 L), Zarrouk medium, under natural light for 30 d (probable stationary phase), and pH 9.8–10.5. | Sugars composition: glucose, galactose, fructose, and organic acids were glucuronic, galacturonic and pyruvic. | 9.5 g L−1 was the highest production of extracellular polymeric substances. | Possible application as a bioflocculant and/or other industrial applications. | [82] |
| Scenedesmus obliquus AS-6-1 | Cultivation to stationary phase, 28 °C, 14/10 h light/dark cycle, 60 mol m−2 s−1. | Cell wall-associated polysaccharides. Monomers consist of glucose, mannose, galactose, rhamnose and fructose. | 0.6 mg L−1 of bioflocculant was responsible for 88% of the flocculant activity of Scenedesmus obliquus FSP-3. | Bioflocculation of Chlorella vulgaris CNW-11, Scenedesmus obliquus FSP-3 and Nannochloropsis oceanica DUT01. | [7] |
| Chlamydomonas reinhardtii | Cultivation at 5 to 25 °C, pH 6–10, 40–60 μmol photons m−2 s−1, 30 d of cultivation. | Bioflocculant composition: proteins (42.1% w w−1), carbohydrates (48.3% w w−1), lipids (8.7% w w−1), and nucleic acid (0.01% w w−1). | 4 mg L−1 of bioflocculant was responsible for 96.6% of the flocculant activity of Chlamydomonas reinhardtii. | Microalgae bioflocculation | [83] |
| Ettlia texensis | Cultivation in a 4 L photobioreactor, batch mode, 24-h lighting, 300 rpm, 26 °C, pH 6.5, and 300 μmol m−2 s−1. | Bioflocculants containing mainly glycoproteins patched to the cell surface. | _ | Microalgae autoflocculation and bioflocculation. | [6] |
| Desmodesmus sp. ZFY and Monoraphidium sp. QLY-1 | Microalgae were used in co-culture, cultivated in mixotrophic medium BG-11 + ammonium nitrate and glucose, pH 6.8, 300 mL, 25 °C, 120 rpm, 3500 lux, and 7 d of cultivation (stationary phase). | Bioflocculant consisted mainly of polysaccharides and proteins. The levels of polysaccharides in co-culture were 46.53% in substances loosely bound to cells (LB-EPS). | Concentration of total extracellular polymeric substances was 368.40 mg L−1. | Microalgae bioflocculation. | [84] |
| Scenedesmus acuminatus | Cultivation performed in 15 L photobioreactors at 25 °C, modified BG-11 medium (NO3 reduction), 180 μmol m−2 s−1, light period 24 h d−1 and pH 6.5–7.0. | High (>50 kDa; 35.1%) and low molecular weight (<3 kDa; 46.1%) polymeric substances were identified; being composed of galactose, glucosamine, mannose. | 3.2 mg g−1 of extracellular polymeric substances were added in the harvesting process together with Al3+ (4.5 mg g−1). | Microalgae bioflocculation. | [10] |
| Chlorella vulgaris JSC-7 | Modified Bold’s Basal Medium with nitrogen supplementation was used, pH 6.9, 28 °C, 13/11 h light/dark cycle and 25 μmol m−2 s−1. | The bioflocculant is a cell wall polysaccharide; The monomers consist of glucose, mannose, and galactose. | 47 mg of the bioflocculant was extracted from 4 L of culture; Addition of 0.5 mg L−1 of the bioflocculant was responsible for >80% of the flocculation of the suspended microalgal cells. | Bioflocculation of C. vulgaris CNW11 and Scenedesmus obliquus FSP. | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.B.; Kuntzler, S.G.; Bezerra, P.Q.M.; Cassuriaga, A.P.A.; Zaparoli, M.; da Silva, J.L.V.; Costa, J.A.V.; de Morais, M.G. Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants. Polysaccharides 2022, 3, 264-276. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3010015
Moreira JB, Kuntzler SG, Bezerra PQM, Cassuriaga APA, Zaparoli M, da Silva JLV, Costa JAV, de Morais MG. Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants. Polysaccharides. 2022; 3(1):264-276. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3010015
Chicago/Turabian StyleMoreira, Juliana Botelho, Suelen Goettems Kuntzler, Priscilla Quenia Muniz Bezerra, Ana Paula Aguiar Cassuriaga, Munise Zaparoli, Jacinta Lutécia Vitorino da Silva, Jorge Alberto Vieira Costa, and Michele Greque de Morais. 2022. "Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants" Polysaccharides 3, no. 1: 264-276. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3010015