Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture
Abstract
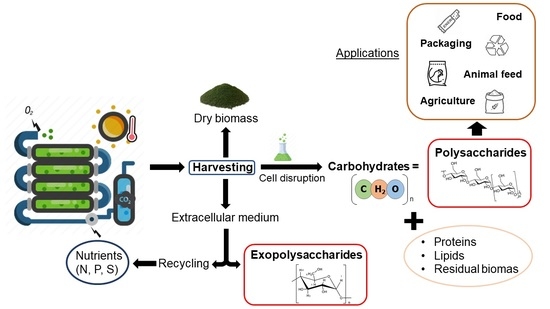
:1. Introduction
2. Microalgae Polysaccharides
2.1. Producing Species and Types of Polysaccharides
2.2. Cultivation Conditions to Increase the Concentration of Polysaccharides
3. Applications of Microalgae Polysaccharides
3.1. Food Sector
3.2. Animal Feed
3.3. Sustainable Agriculture
4. Future Work and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, J.B.; Santos, T.D.; Duarte, J.H.; Bezerra, P.Q.M.; de Morais, M.G.; Costa, J.A.V. Role of microalgae in circular bioeconomy: From waste treatment to biofuel production. Clean Techno.l Environ. Policy 2021, 1–11. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Moreira, J.B.; Fanka, L.S.; Kosinski, R.D.C.; de Morais, M.G. Microalgal biotechnology applied in biomedicine. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 429–439. [Google Scholar]
- Braga, V.D.S.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Enhancement of the carbohydrate content in Spirulina by applying CO2, thermoelectric fly ashes and reduced nitrogen supply. Int. J. Biol. Macromol. 2019, 123, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Braga, V.D.S.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Potential of Chlorella fusca LEB 111 cultivated with thermoelectric fly ashes, carbon dioxide and reduced supply of nitrogen to produce macromolecules. Bioresour. Technol. 2019, 277, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Carraro, C.F.F.; Loures, C.C.A.; Castro, J.A. Microalgae biorremediation and CO2 fixation of industrial waster. Clean. Technol. 2022, 8, 100466. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Moraes, L.; Moreira, J.B.; Rosa, G.M.; Henrard, A.S.A.; Morais, M.G. Microalgae-based biorefineries as a promising approach to biofuel production. In Prospects and Challenges in Algal Biotechnology; Tripathi, B.N., Kumar, D., Eds.; Springer: Singapore, 2017; pp. 113–140. [Google Scholar]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatt, M.; Singh, J. Potential biotechnological applications of cyanobacterial exopolysaccharides. Braz. Arch. Biol. Technol. 2021, 64, e21200401. [Google Scholar] [CrossRef]
- Colusse, G.A.; Carneiro, J.; Duarte, M.E.R.; De Carvalho, J.C.; Noseda, M.D. Advances in microalgal cell wall polysaccharides: A review focused on structure, production, and biological application. Crit. Rev. Biotechnol. 2021, 41, 1–16. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [Green Version]
- Morais, M.G.; Santos, T.D.; Moraes, L.; Vaz, B.S.; Morais, E.G.; Costa, J.A. Exopolysaccharides from microalgae: Production in a biorefinery framework and potential applications. Bioresour. Technol. Rep. 2022, 18, 101006. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Bioactive polysaccharides and their derivatives from microalgae: Biosynthesis, applications, and challenges. Stud. Nat. Prod. Chem. 2021, 71, 67–85. [Google Scholar]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.J.; Chen, C.W.; Dong, C.D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2022, 48, 451–463. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Malyar, Y.N.; Issaoui, N.; Vasilieva, N.Y.; Karacharov, A.A. Synthesis optimization, DFT and physicochemical study of chitosan sulfates. J. Mol. Struct. 2021, 1245, 131083. [Google Scholar] [CrossRef]
- Chanda, M.; Merghoub, N.; Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Moreira, J.B.; Kuntzler, S.G.; Bezerra, P.Q.M.; Cassuriaga, A.P.A.; Zaparoli, M.; da Silva, J.L.V.; Costa, J.A.V.; da Morais, M.G. Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants. Polysaccharides 2022, 3, 264–276. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Prybylski, N.; Toucheteau, C.; El Alaoui, H.; Bridiau, N.; Maugard, T.; Abdelkafi, S.; Fendri, I.; Delattre, C.; Dubessay, P.; Pierre, G.; et al. Bioactive polysaccharides from microalgae. In Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Academic Press: Cambridge, MA, USA, 2020; pp. 533–571. [Google Scholar]
- Silvello, M.A.D.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef]
- Keidan, M.; Friedlander, M.; Arad, S. Effect of Brefeldin A on cell-wall polysaccharide production in the red microalga Porphyridium sp. (Rhodophyta) through its effect on the Golgi apparatus. J. Appl. Phycol. 2009, 21, 707–717. [Google Scholar] [CrossRef]
- Raposo, M.F.; Morais, R.M.S.C.; Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Morais, M.G.; Rosa, G.M.; Moraes, L.; Alvarenga, A.G.P.; Silva, J.L.V.; Costa, J.A.V. Microalgae Polysaccharides with Potential Biomedical Application. In Polysaccharides of Microbial Origin; Springer International Publishing: Cham, Switzerland, 2021; pp. 363–380. [Google Scholar]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health Benefits of Algal Polysaccharides in Human Nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Deamici, K.; de Morais, M.; Santos, L.; Muylaert, K.; Gardarin, C.; Costa, J.; Laroche, C. Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains. Appl. Sci. 2021, 11, 5299. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: From algae to flowering plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.; Horst, G.; Tonda, R.; Lumpkins, B.; Mathis, G. Evaluation of the effects of feeding dried algae containing beta-1,3-glucan on broilers challenged with Eimeria. Poult. Sci. 2018, 97, 3494–3500. [Google Scholar] [CrossRef]
- Iwamoto, H. Industrial production of microalgal cell-mass and secondary products—Major Industrial Species: Chlorella. Handb. Microalgal Cult. 2004, 255, 253–263. [Google Scholar]
- Ravindran, R.; Rajauria, G. Carbohydrates derived from microalgae in the food industry. Cult. Microalgae Food Ind. 2021, 127–146. [Google Scholar] [CrossRef]
- Rossi, F.; Philips, R.D. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae; Borowtzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 565–590. [Google Scholar]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Koçer, A.T.; Inan, B.; Usul, S.K.; Özçimen, D.; Yılmaz, M.T.; Işıldak, I. Exopolysaccharides from microalgae: Production, characterization, optimization and techno-economic assessment. Braz. J. Microbiol. 2021, 52, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, S.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2022, 309, 123362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, isolation and bioactive estimation of extracellular polysaccharides of green microalga Neochloris oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Optimization of growth and EPS production in two Porphyridum strains. Bioresour. Technol. Rep. 2020, 11, 100486. [Google Scholar] [CrossRef]
- Cepák, V.; Přibyl, P. Light intensity and nitrogen effectively control exopolysaccharide production by the green microalga Botryococcus Braunii (Trebouxiophyceae). Genet. Plant. Physiol. 2018, 8, 24–37. [Google Scholar]
- Gui, J.; Tong, W.; Huang, S.; Liang, X.; Fang, Z.; Wang, W.; Zhang, Y. Effects of Chlorella vulgaris polysaccharides accumulation on growth characteristics of Trachemys scripta elegans. Int. J. Biol. Macromol. 2019, 141, 1304–1313. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Liang, H.; Xue, S. Selective carbon sources and salinities enhance enzymes and extracellular polymeric substances extrusion of Chlorella sp. for potential co-metabolism. Bioresour. Technol. 2020, 303, 122877. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; Sadok, A.D.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Mandik, Y.I.; Prasertsan, P. Evaluation of optimal conditions for cultivation of marine Chlorella sp. as potential sources of lipids, exopolymeric substances and pigments. Aquac. Int. 2016, 24, 313–326. [Google Scholar] [CrossRef]
- Díaz-Bayona, K.C.; Garcés, L.A. Effect of different media on exopolysaccharide and biomass production by the green microalga Botryococcus braunii. J. Appl. Phycol. 2014, 26, 2087–2095. [Google Scholar] [CrossRef]
- Turpin, D.H. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J. Phycol. 1991, 27, 14–20. [Google Scholar] [CrossRef]
- Bellou, S.; Aggelis, G. Biochemical activities in Chlorella sp. and Nannochloropsis salina during lipid and sugar synthesis in a lab scale open pond simulating reactor. J. Biotechnol. 2013, 164, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Kumar, G. Investigation of four microalgae in nitrogen deficient synthetic wastewater for biorefinery based biofuel production. Environ. Technol. Innov. 2021, 23, 101572. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: Improvements through phosphorus limitation process. BioEnergy Res. 2012, 5, 915–925. [Google Scholar] [CrossRef]
- Solís-Salinas, C.E.; Patlán-Juárez, G.; Okoye, P.U.; Guillén-Garcés, A.; Sebastian, P.J.; Arías, D.M. Long-term semi-continuous production of carbohydrate-enriched microalgae biomass cultivated in low-loaded domestic wastewater. Sci. Total Environ. 2021, 798, 149227. [Google Scholar] [CrossRef]
- Morais, M.G.; Morais, E.G.; Cardias, B.C.; Vaz, B.S.; Moreira, J.B.; Mitchell, G.; Costa, J.A.V. Microalgae as a source of sustainable biofuels. In Recent Developments In Bioenergy Research; Gupta, V.K., Treichel, H., Rodriguez-Cout, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 253–271. [Google Scholar]
- Chen, L.Z.; Li, D.-H.; Song, L.R.; Hu, C.X.; Wang, G.H.; Liu, Y.D. Effects of salt stress on carbohydrate metabolism in desert soil alga Microcoleus vaginatus. Gom. J. Integr. Plant Biol 2006, 48, 914–919. [Google Scholar] [CrossRef]
- Champigny, M. Regulation of photosynthetic carbon assimilation at the cellular level: A review. Photosynth. Res. 1985, 6, 273–286. [Google Scholar] [CrossRef]
- Vonshak, A. Spirulina Platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; Taylor and Francis: London, UK, 1997; 233p. [Google Scholar]
- Zhao, T.; Han, X.; Cao, H. Effect of Temperature on Biological Macromolecules of Three Microalgae and Application of FT-IR for Evaluating Microalgal Lipid Characterization. ACS Omega 2020, 5, 33262–33268. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef]
- Medeiros, V.P.B.; Souza, E.L.; Albuquerque, T.M.R.; Sassi, C.F.C.; Lima, M.S.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Tiwaria, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.K.; Jaladid, P.; Oiname, G.; Indrama, T. Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Arad, S.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Jindal, N.; Singh, D.P.; Khattar, J.I.S. Optimization, characterization, and flow properties of exopolysaccharides produced by the cyanobacterium Lyngbya stagnina. J. Basic Microbiol. 2013, 53, 902–912. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Mun, S.C.; Chang, Y.K.; Choi, S.Q. A new method to produce cellulose nanofibrils from microalgae and the measurement of their mechanical strength. Carbohydr. Polym. 2018, 180, 276–285. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Torzillo, G.; De Philippis, R. Exopolysaccharide features influence growth success in biocrust-forming cyanobacteria, moving from liquid culture to sand microcosms. Front. Microbiol. 2020, 11, 568224. [Google Scholar] [CrossRef]
- Park, C.H.; Li, X.R.; Zhao, Y.; Jia, R.L.; Hur, J.S. Rapid development of cyanobacterial crust in the field for combating desertification. PLoS One 2017, 12, e0179903. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Rossi, F.; Colica, G.; Deng, S.; Philippis, R.; Chen, L. Use of cyanobacterial polysaccharides to promote shrub performances in desert soils: A potential approach for the restoration of desertified areas. Biol. Fertil. Soils. 2013, 49, 143–152. [Google Scholar] [CrossRef]
- Li, T.T.; Huang, Z.R.; Jia, R.B.; Lv, X.C.; Zhao, C.; Liu, B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Rajasekar, P.; Palanisamy, S.; Anjali, R.; Vinosha, M.; Elakkiya, M.; Marudhupandi, T.; Tabarsa, M.; You, S.; Prabhu, N.M. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int. J. Biol. Macromol. 2019, 141, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Lin, A.P.; Sun, Y.; Deng, Y.M. Chemo- and radio-protective effects of polysaccharide of Spirulina platensis on hemopoietic system of mice and dogs. Acta Pharmacol. Sin. 2001, 22, 1121–1124. [Google Scholar] [PubMed]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as sustainable food and feed sources for animals and humans e Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a Potential Natural Functional Food Source—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Gouda, M.; Tadda, M.A.; Zhao, Y.; Farmanullah, F.; Chu, B.; Li, X.; He, Y. Microalgae Bioactive Carbohydrates as a Novel Sustainable and Eco-Friendly Source of Prebiotics: Emerging Health Functionality and Recent Technologies for Extraction and Detection. Front. Nutr. 2022, 9, 806692. [Google Scholar] [CrossRef]
- Venugopal, V. Marine Polysaccharides: Food Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; 396p. [Google Scholar]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Heo, M.-G.; Choung, S.-Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-obesity effects of microalgae. Int. J. Mol. Sci. 2019, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorates obesity in association with modulation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
- Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Hamed, I.; Jakobsen, A.N.; Lerfall, J. Sustainable edible packaging systems based on active compounds from food processing byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.M.; Selvakumar, R.; Suresh, K.P.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P.M. Microalgae as Contributors to Produce Biopolymers. Mar. Drugs 2021, 19, 466. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Devi, M.A.; Venkataraman, L.V. Supplementary value of the proteins of blue green algae Spirulina platensis to rice and wheat proteins. Nutr. Rep. Int. 1983, 28, 1029–1035. [Google Scholar]
- Dawood, M.A.; Moustafa, E.M.; Elbialy, Z.I.; Farrag, F.; Lolo, E.E.; Abdel-Daim, H.A.; Van Doan, H. Lactobacillus plantarum L-137 and/or β-glucan impacted the histopathological, antioxidant, immune-related genes and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Res. Vet. Sci. 2020, 130, 212–221. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef] [Green Version]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M.Á. Increases in immune parameters by inulin and Bacillus subtilis dietary administration to gilthead seabream (Sparus aurata L.) did not correlate with disease resistance to Photobacterium damselae. Fish Shellfish Immunol. 2012, 32, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, M.; Rengasamy, R. Antioxidant status of Penaeus monodon fed with Dunaliella salina supplemented diet and resistance against WSSV. Int. J. Eng. Sci. Technol. 2011, 3, 7249–7259. [Google Scholar]
- Nath, P.R.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.; Zilberg, D. Dietary supplementation with the microalgae Parietochloris incisa increases survival and stress resistance in guppy (Poecilia reticulata) fry. Aquac. Nutr. 2012, 18, 167–180. [Google Scholar] [CrossRef]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J. Environ. Sci. Health Part B 2019, 54, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Stirk, W.A.; Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef]
- Guzmán-Murillo, M.A.; Ascencio, F.; Larrinaga-Mayoral, J.A. Germination and ROS detoxification in bell pepper (Capsicum annuum L.) under NaCl stress and treatment with microalgae extracts. Protoplasma 2013, 250, 33–42. [Google Scholar] [CrossRef]
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Takagi, A.; Ota, S.; Kawano, S.; Sasaki, D.; Asayama, M. Coproduction of lipids and extracellular polysaccharides from the novel green alga Parachlorella sp. BX1.5 depending on cultivation conditions. Biotechnol. Rep. 2020, 25, e00392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Han, X.; Qiu, Y.; Liu, Z.; Li, S.; Kitamura, Y. Microalgae carbon fixation integrated with organic matters recycling from soybean wastewater: Effect of pH on the performance of hybrid system. Chemosphere 2020, 248, 126094. [Google Scholar] [CrossRef] [PubMed]


| Monosaccharides and polysaccharides | Chemical Structure | Source | Reference |
|---|---|---|---|
| Glucose (C6H12O6) |  α-D-Glucose β-D-Glucose | EPS from cyanobacteria | [16] |
| Fucose (C6H12O5) |  D-Fucose L-Fucose | EPS from Charophyta | [16] |
| Uronic acids (Cn(H2O)n) |  Manuronic acid Galacturonic acid Glucuronic acid | EPS from Charophyta | [16] |
| Xylose (C5H10O5) |  D-Xylose L-Xylose | EPS from Rhodophyta and cyanobacteria | [16] |
| Galactose (C6H12O6) |  D-galactose L-Galactose | EPS from Rhodophyta, Chlorophyta and cyanobacteria | [16] |
| Cellulose (C6H10O5)n |  | Cell wall of microalgae | [30] |
| Hemicellulose (C5H8O4)n or (C6H10O5)n |  | Cell wall of microalgae | [30] |
| β-glucan (C18H32O16) |  | Euglena gracilis and Chlorella sp. | [31,32] |
| Microalga | Culture Medium | Cultivation Conditions | Substrate | Results | Reference |
|---|---|---|---|---|---|
| Chlorella minutissima, Chlorella sorokiniana and Botryococcus braunii | BG-11 medium | Carbon (Na2CO3) and nitrogen (NaNO3) content in the culture medium | - | Lower concentrations of Na2CO3 (0.02 g L−1) and (NaNO3) (0.2 g L−1) promoted higher EPS production in Chlorella minutissima (0.245 g L−1), Chlorella sorokiniana (0.163 g L−1) and Botryococcus braunii (0.117g L−1) | [36] |
| Neochloris oleoabundans | Modified medium SE | Carbon/nitrogen (C/N) ratio, nitrogen limitation | Glucose, galactose, maltose, lactose and sucrose | The use of 20 g L−1 of glucose and 12 mM of NaNO3 (low N concentration) were favorable to the production of polysaccharides. | [37] |
| Porphyridium purpureum | ASW medium with low carbon/nitrogen ratio (LC/N), medium carbon/nitrogen ratio (MC/N) and high carbon/nitrogen ratio (HC/N) | C/N ratio | - | HC/N and MC/N resulted in higher EPS production (around 1.75 g L−1). Higher C/N ratios stimulated the production of this molecule. | [38] |
| Porphyridium sordidum and Porphyridium purpureum | Artificial sea water | Different wavelengths—blue light (430 nm); combination of lights green/yellow/orange (572/625/640 nm), orange/red (660/780 nm) and white light (combination of all) | - | White light was the most suitable for the production of polysaccharides (Porphyridium sordidum (0.10 g L−1) and Porphyridium purpureum (0.14 g L−1) | [39] |
| Botryococcus braunii | Zehnder medium | Light intensity and nitrogen concentration | - | Higher production of polysaccharides at higher light intensities (650–950 μmol m−2 s−1) and nitrogen concentration of 6 mM, supplied as potassium nitrate. | [40] |
| Chlorella vulgaris | BG-11 medium | Light intensity and temperature | - | Accumulation of 32.7% of polysaccharides under the conditions of 65 μmol m−2 s−1 and 28 °C. | [41] |
| Chlorella sp. | MLA medium | Different sources of carbon and salinity | Methanol, ethanol, sucrose, glucose, sodium acetate, glycine, sodium bicarbonate. | Higher production of polysaccharides (0.01g L−1) with microalgae cultured with glucose and sucrose. When increasing salinity from 0.1% to 3.5%, EPS concentrations increased 2-fold. | [42] |
| Spirulina sp. | Zarrouk médium | Light intensity and NaCl concentration | - | Light intensity did not affect polysaccharide production. High concentration of NaCl (40 g L−1) increased the production of EPS (1.02 g g−1 of biomass). | [43] |
| Chlorella sp. | BG-11 medium | Light intensity. Mixotrophic cultivation | Glucose | Light intensity of 65 µmol m−2 s−1 and glucose concentration of up to 1% w v−1 improved EPS yields (1.46 g L−1) in semi-continuous culture. | [44] |
| Botryococcus braunii | BG-11 and D medium | Influence of nitrogen concentration and salinity of culture media | - | EPS production was higher in medium D (0.549 g L−1) than in BG11 (0.336 g L−1). Influence of lower N concentration and higher salinity of medium D (NaNO3 = 0.689 g L−1 and NaCl = 0.008 g L−1) | [45] |
| Microalgae | Application | Polysaccharide | Main Results | Reference |
|---|---|---|---|---|
| Arthrospira platensis, Chlorella vulgaris | Food sector | Native and modified forms of polysaccharides as dietary prebiotics | Prebiotic score was 6.93 ± 0.05 and Chlorella vulgaris was 2.54 ± 0.02, results significantly high compared to the control (−1.35 ± 0.04). | [58] |
| Anabaena sp. CCC 745 | Food sector | Cyanobacteria exopolysaccharides | Exopolysaccharides exhibited pseudoplastic fluid behavior, and significant antioxidant and scavenging activity. | [59] |
| Porfyridium sp. | Food sector | Exopolysaccharides of red microalga | Exopolysaccharides showed intrinsic viscosity higher than the values reported in the literature for hydrocolloids. | [60] |
| Lyngbya stagnina | Food sector | Cyanobacteria exopolysaccharides | Exopolysaccharides showed non-Newtonian behavior, pseudoplastic, and stable viscosity. Results similar to commercial Xanthan gum. | [61] |
| Nannochloropsis oceanica | Food sector | Cellulose nanofibrils from Nannochloropsis | Cellulose nanofibrils with 3–4 GPA tensile strength, being similar or higher than other general packaging reinforcements currently applied. | [62] |
| Arthrospira platensis, Dunaliella salina, Porphyridium sp. | Agriculture | Polysaccharides extracts used for irrigation | Polysaccharides extracts in tomato plants improved significantly the nodes number, shoot dry weight, and shoot length compared to control. | [63] |
| Nostoc commune, Scytonema javanicum, and Phormidium ambiguum | Agriculture | Cyanobacteria exopolysaccharides | Polysaccharidic matrix produced by different cyanobacteria can influence their growth in soil. | [64] |
| Nostoc sp., Phormidium sp., and Scytonema arcangeli | Agriculture | Tacki-SprayTM (TKS7), which consists of bio-polysaccharides and tackifiers, was used as a soil fixing agent. | Combined application of cyanobacteria with soil fixing chemicals can rapidly develop cyanobacterial crust formation in the field within 12 months. | [65] |
| Phormidium tenue | Agriculture | Crude polysaccharide synthesized by Phormidium tenue | The polysaccharide significantly increased seed germination and metabolic activity of the seedling of the shrub Caragana korshinskii. | [66] |
| Spirulina platensis | Animal feed | Spirulina platensis crude polysaccharides | Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats. | [67] |
| Spirulina platensis | Animal feed | Sulfated polysaccharide from Spirulina platensis | In vitro antioxidant activity, antibacterial activity, and Zebrafish growth and reproductive performance. | [68] |
| Chlorella pyrenoidosa | Animal feed | Chlorella pyrenoidosa polysaccharides | Chlorella pyrenoidosa polysaccharides levels had a positive effect on the specific growth rate of the Trachemys scripta elegans. | [69] |
| Spirulina platensis | Animal feed | Polysaccharide of Spirulina platensis | Chemo- and radio-protective effects of polysaccharide of Spirulina platensis on hemopoietic system of mice and dogs. | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.B.; Vaz, B.d.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.d.; Costa, J.A.V.; Morais, M.G.d. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441-457. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020027
Moreira JB, Vaz BdS, Cardias BB, Cruz CG, Almeida ACAd, Costa JAV, Morais MGd. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides. 2022; 3(2):441-457. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020027
Chicago/Turabian StyleMoreira, Juliana Botelho, Bruna da Silva Vaz, Bruna Barcelos Cardias, Camila Gonzales Cruz, Ana Claudia Araujo de Almeida, Jorge Alberto Vieira Costa, and Michele Greque de Morais. 2022. "Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture" Polysaccharides 3, no. 2: 441-457. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020027