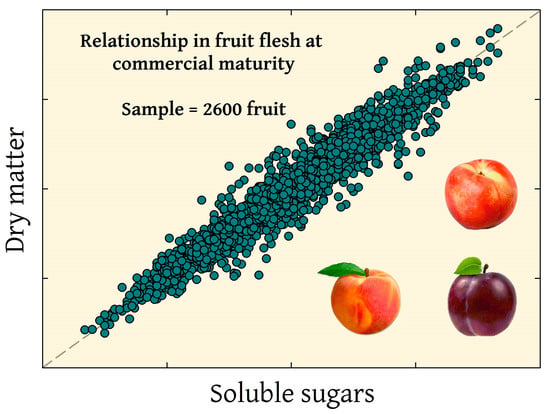
Relationships between Soluble Solids and Dry Matter in the Flesh of Stone Fruit at Harvest
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, C.; Crisosto, G.M.; Heymann, H.; Crisosto, C.H. Determining the Primary Drivers of Liking to Predict Consumers’ Acceptance of Fresh Nectarines and Peaches. J. Food Sci. 2013, 78. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, G.; Echeverria, G.; Bellvert, J.; Mata, M.; Behboudian, M.H.; Girona, J.; Marsal, J. Water stress for a short period before harvest in nectarine: Yield, fruit composition, sensory quality, and consumer acceptance of fruit. Sci. Hortic. (Amsterdam) 2016, 211. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Lopresti, J.; Goodwin, I.; McGlasson, B.; Holford, P.; Golding, J. Variability in size and soluble solids concentration in peaches and nectarines. Hortic. Rev. (Am. Soc. Hortic. Sci.) 2014, 42, 253–311. [Google Scholar] [CrossRef]
- Lopresti, J.; Goodwin, I.; Stefanelli, D.; Holford, P.; McGlasson, B.; Golding, J. Understanding the factors affecting within-tree variation in soluble solids concentration in peaches and nectarines. Acta Hortic. 2016, 1130, 249–256. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [Green Version]
- Crisosto, C.H. Stone fruit maturity indices: A descriptive review. Postharvest News Inf. 1994, 5, 65N–68N. [Google Scholar]
- Subedi, P.P.; Walsh, K.B.; Owens, G. Prediction of mango eating quality at harvest using short-wave near infrared spectrometry. Postharvest Biol. Technol. 2007, 43. [Google Scholar] [CrossRef]
- Gamble, J.; Harker, F.R.; Jaeger, S.R.; White, A.; Bava, C.; Beresford, M.; Stubbings, B.; Wohlers, M.; Hofman, P.J.; Marques, R.; et al. The impact of dry matter, ripeness and internal defects on consumer perceptions of avocado quality and intentions to purchase. Postharvest Biol. Technol. 2010, 57. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Day, K.R. Stone Fruit. In Crop Post-Harvest: Science and Technology; Rees, D., Farrell, G., Orchard, J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 212–225. ISBN 9780632057252. [Google Scholar]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Clark, C.J. Dry-matter—A better predictor of the post-storage soluble solids in apples? Postharvest Biol. Technol. 2003, 28. [Google Scholar] [CrossRef]
- Palmer, J.W.; Harker, F.R.; Tustin, D.S.; Johnston, J. Fruit dry matter concentration: A new quality metric for apples. J. Sci. Food Agric. 2010. [Google Scholar] [CrossRef] [PubMed]
- Goke, A.; Serra, S.; Musacchi, S. Postharvest dry matter and soluble solids content prediction in d’anjou and bartlett pear using near-infrared spectroscopy. HortScience 2018. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Lo Bianco, R. Field non-destructive determination of nectarine quality under deficit irrigation. Acta Hortic. accepted.
- Wills, R.B.H.; Scriven, F.M.; Greenfield, H. Nutrient composition of stone fruit (Prunus spp.) cultivars: Apricot, cherry, nectarine, peach and plum. J. Sci. Food Agric. 1983, 34, 1383–1389. [Google Scholar] [CrossRef]
- Jones, G.P.; Briggs, D.R.; Wahlquvist, L.M.; Flentje, S.B.J. Dietary fibre content of Australian foods. 3. Fruits and fruit products. Food Aust. 1990, 42, 143–145. [Google Scholar]
- Quilot, B.; Kervella, J.; Génard, M. Shape, mass and dry matter content of peaches of varieties with different domestication levels. Sci. Hortic. (Amsterdam) 2004, 99. [Google Scholar] [CrossRef]
- Lintas, C.; Cappelloni, M. Dietary fiber content of italian fruit and nuts. J. Food Compos. Anal. 1992, 5, 146–151. [Google Scholar] [CrossRef]
- Englyst, H.N.; Bingham, S.A.; Runswick, S.A.; Collinson, E.; Cummings, J.H. Dietary fibre (non-starch polysaccharides) in fruit, vegetables and nuts. J. Hum. Nutr. Diet. 1988, 1, 247–286. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Wu, B.; Quilot, B.; Kervella, J.; Génard, M.; Li, S. Analysis of genotypic variation of sugar and acid contents in peaches and nectarines through the Principle Component Analysis. Euphytica 2003, 132. [Google Scholar] [CrossRef]
- Peiris, K.H.S.; Dull, G.G.; Leffler, R.G.; Kays, S.J. Spatial variability of soluble solids or dry-matter content within individual fruits, bulbs, or tubers: Implications for the development and use of NIR spectrometric techniques. HortScience 1999, 34. [Google Scholar] [CrossRef] [Green Version]
- Kobashi, K.; Gemma, H.; Iwahori, S. Abscisic acid content and sugar metabolism of peaches grown under water stress. J. Am. Soc. Hortic. Sci. 2000, 125, 425–428. [Google Scholar] [CrossRef]
- Naor, A. Irrigation Scheduling and Evaluation of Tree Water Status in Deciduous Orchards. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 111–166. ISBN 9780470767986. [Google Scholar]
- Mills, T.M.; Behboudian, M.H.; Tan, P.Y.; Clothier, B.E. Plant water status and fruit quality in Braeburn’ apples. HortScience 1994, 29. [Google Scholar] [CrossRef] [Green Version]
- Kilili, A.W.; Behboudian, M.H.; Mills, T.M. Composition and quality of ‘Braeburn’ apples under reduced irrigation. Sci. Hortic. (Amsterdam) 1996, 67. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Behboudian, M.H.; Green, S.R. Water use, yield and fruit quality of lysimeter-grown apple trees: Responses to deficit irrigation and to crop load. Irrig. Sci. 2001, 20. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Socquet-Juglard, D.; Christen, D.; Devènes, G.; Gessler, C.; Duffy, B.; Patocchi, A. Mapping Architectural, Phenological, and Fruit Quality QTLs in Apricot. Plant Mol. Biol. Report. 2013, 31. [Google Scholar] [CrossRef]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Valero, C.; Momin, M.A.; Kaur, A.; Slaughter, D.C. Performance evaluation of two commercially available portable spectrometers to non-invasively determine table grape and peach quality attributes. Agronomy 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Azodanlou, R.; Darbellay, C.; Luisier, J.L.; Villettaz, J.C.; Amadò, R. Development of a model for quality assessment of tomatoes and apricots. LWT Food Sci. Technol. 2003, 36, 223–233. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. (Amsterdam) 2009, 120, 41–50. [Google Scholar] [CrossRef]
- O’Connell, M.; Stefanelli, D. Effect of crop load management and canopy architecture on yield and fruit quality of late-season plum ‘Angeleno’. Acta Hortic. 2020, 1281, 227–233. [Google Scholar] [CrossRef]
- O’Connell, M.; Stefanelli, D. Effects of rootstock and crop load management on yield and fruit quality of early-season nectarine ‘Rose Bright’ and late-season peach ‘September Sun’. Acta Hortic. 2020, 1281, 121–129. [Google Scholar] [CrossRef]
- O’Connell, M.G.; Scalisi, A. Sensing fruit and tree performance under deficit irrigation in ‘September Bright’ nectarine. Acta Hortic. accepted.




| Crop | Cultivar | Best-Fit Linear Regression Coefficients | R2 | ANOVA | RMSE for Best-Fit Model | RMSE for DMC = SSC Model | ΔRMSE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Intercept SE | Slope | Slope SE | Intercept (p) | Slope (p) | ||||||
| Nectarine | ‘August Bright’ | 1.92 | 0.31 | 0.87 | 0.02 | 0.899 | <0.05 | <0.05 | 0.747 | 0.829 | 0.082 |
| ‘Autumn Bright’ | 0.19 | 0.18 | 0.99 | 0.02 | 0.952 | 0.305 | 0.527 | 0.377 | 0.385 | 0.008 | |
| ‘Rose Bright’ | 2.09 | 0.29 | 0.85 | 0.02 | 0.888 | <0.05 | <0.05 | 0.710 | 0.798 | 0.088 | |
| ‘September Bright’ | 0.25 | 1.03 | 0.02 | 0.952 | 0.050 | 0.061 | 0.502 | 0.507 | 0.005 | ||
| Yellow peach | ‘August Flame’ | 2.25 | 0.31 | 0.81 | 0.02 | 0.862 | <0.05 | <0.05 | 0.726 | 0.918 | 0.192 |
| ‘O’Henry’ | 2.58 | 0.35 | 0.82 | 0.02 | 0.868 | <0.05 | <0.05 | 0.748 | 0.854 | 0.106 | |
| ‘Redhaven’ | 1.25 | 0.39 | 0.88 | 0.03 | 0.778 | <0.05 | <0.05 | 0.621 | 0.657 | 0.036 | |
| ‘September Sun’ | 1.18 | 0.25 | 0.95 | 0.02 | 0.933 | <0.05 | <0.05 | 0.567 | 0.818 | 0.251 | |
| White peach | ‘Ice Princess’ | 1.28 | 0.29 | 0.91 | 0.02 | 0.900 | <0.05 | <0.05 | 0.746 | 0.782 | 0.036 |
| ‘Snow Fall’ | 1.16 | 0.43 | 0.98 | 0.02 | 0.894 | <0.05 | 0.351 | 0.642 | 0.998 | 0.356 | |
| ‘Snow Flame 23’ | 1.93 | 0.31 | 0.88 | 0.02 | 0.900 | <0.05 | <0.05 | 0.684 | 0.752 | 0.068 | |
| ‘Snow Flame 25’ | 3.04 | 0.41 | 0.81 | 0.03 | 0.835 | <0.05 | <0.05 | 0.638 | 0.720 | 0.082 | |
| Apricot | ‘Golden May’ | 5.72 | 0.32 | 0.55 | 0.03 | 0.699 | <0.05 | <0.05 | 1.328 | 2.144 | 0.816 |
| Plum | ‘Angeleno’ | 0.46 | 0.40 | 0.96 | 0.02 | 0.918 | 0.254 | 0.074 | 0.388 | 0.470 | 0.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalisi, A.; O’Connell, M.G. Relationships between Soluble Solids and Dry Matter in the Flesh of Stone Fruit at Harvest. Analytica 2021, 2, 14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica2010002
Scalisi A, O’Connell MG. Relationships between Soluble Solids and Dry Matter in the Flesh of Stone Fruit at Harvest. Analytica. 2021; 2(1):14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica2010002
Chicago/Turabian StyleScalisi, Alessio, and Mark Glenn O’Connell. 2021. "Relationships between Soluble Solids and Dry Matter in the Flesh of Stone Fruit at Harvest" Analytica 2, no. 1: 14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica2010002