Influence of Moderate Cd and Pb Soil Pollution on Seed Development, Photosynthetic Performance and Foliar Accumulation in the Medicinal Plant Hypericum perforatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Experiment
2.1.1. Seed Collection
2.1.2. Seed Pre-Treatment and Exposure
2.2. Plant Experiment
2.2.1. Plant Growth
2.2.2. Plant Exposure
2.2.3. Physiological Parameters
2.2.4. Accumulation of Cd and Pb
2.3. Statistical Analysis
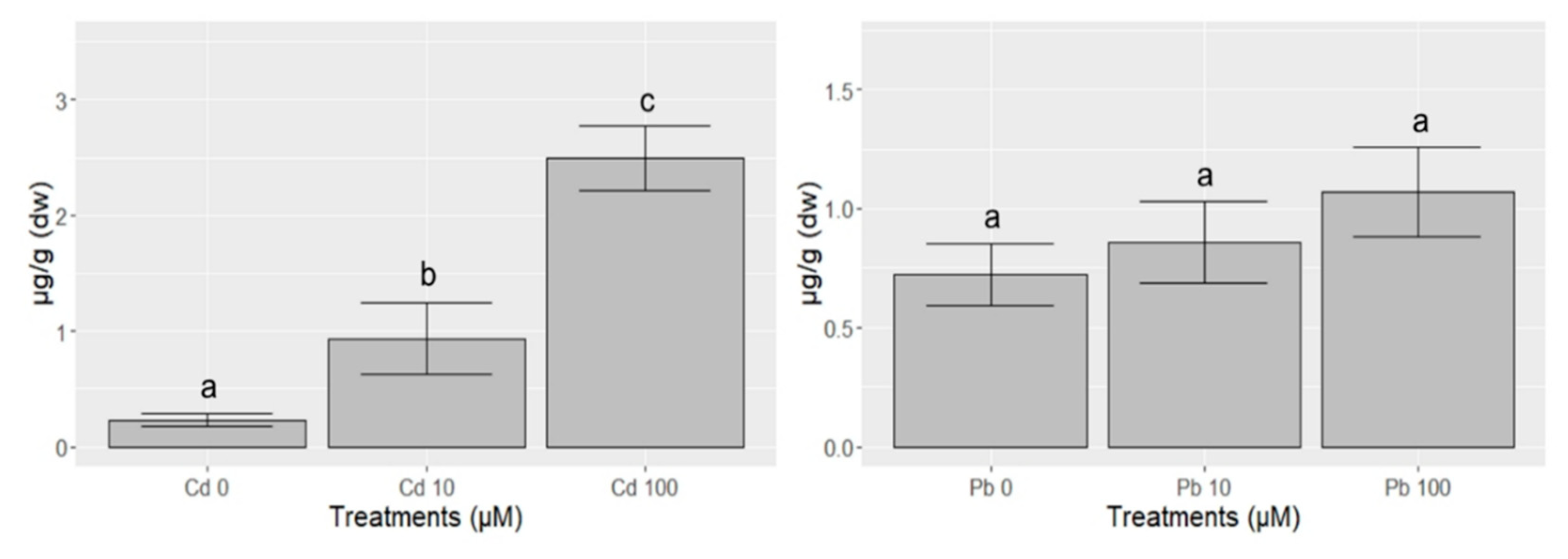
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Environmental Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10 (accessed on 4 September 2020).
- Aarhus Protocol on Heavy Metals. 1998. Available online: http://www.unece.org/env/lrtap/hm_h1.html (accessed on 4 September 2020).
- Pacyna, E.G.; Pacyna, J.M.; Fudala, J.; Strzelecka-Jastrzab, E.; Hlawiczka, S.; Panasiuk, D.; Nitter, S.; Pregger, T.; Pfeiffer, H.; Friedrich, R. Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos. Environ. 2007, 41, 8557–8566. [Google Scholar] [CrossRef]
- Wu, J.; Boyle, E.A. Lead in the western North Atlantic Ocean: Completed response to leaded gasoline phaseout. Geochim. Cosmochim. Acta 1997, 61, 3279–3283. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Alloway, B.J.; Steinnes, E. Anthropogenic Additions of Cadmium to Soils. In Cadmium in Soils and Plants; Springer: Dordrecht, The Netherlands, 1999; pp. 97–123. [Google Scholar]
- Markus, J.; McBratney, A.B. A review of the contamination of soil with lead II. Spatial distribution and risk assessment of soil lead. Environ. Int. 2001, 27, 399–411. [Google Scholar] [CrossRef]
- Farooqi, Z.R.; Iqbal, M.Z.; Kabir, M.; Shafiq, M. Toxic effects of lead and cadmium on germination and seedling growth of Albizia lebbeck (L.) Benth. Pak. J. Bot. 2009, 41, 27–33. [Google Scholar]
- Fargašová, A. Effect of Pb, Cd, Hg, As, and Cr on germination and root growth of Sinapis alba seeds. Bull. Environ. Contam. Toxicol. 1994, 52, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Iqbal, M.; Mohammad, A. Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. J. Appl. Sci. Environ. Manag. 2010, 12. [Google Scholar] [CrossRef] [Green Version]
- Seregin, I.V.; Ivanov, V.B. Physiological Aspects of Cadmium and Lead Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Aery, N.C.; Jagetiya, B.L. Relative toxicity of cadmium, lead, and zinc on barley. Commun. Soil Sci. Plant Anal. 1997, 28, 949–960. [Google Scholar] [CrossRef]
- Breckle, S.-W.; Kahle, H. Effects of toxic heavy metals (Cd, Pb) on growth and mineral nutrition of beech (Fagus sylvatica L.). Vegetatio 1992, 101, 43–53. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Cadmium and lead-induced changes in lipid peroxidation, antioxidative enzymes and metal accumulation in Brassica juncea L. at three different growth stages. Arch. Agron. Soil. Sci. 2009, 55, 395–405. [Google Scholar] [CrossRef]
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. 11 Medical Attributes of St. John’s Wort (Hypericum perforatum). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, R.A. Heavy metals in medicinal plant products—An African perspective. S. Afr. J. Bot. 2012, 82, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Zarinkamar, F.; Ghelich, S.; Soleimanpour, S. Toxic Effects of Pb on Anatomy and Hypericin Content in Hypericum perforatum L. Bioremediat. J. 2013, 17, 40–51. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- Chizzola, R.; Lukas, B. Variability of the Cadmium Content in Hypericum Species Collected in Eastern Austria. Water Air Soil Pollut. 2006, 170, 331–343. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Peñalosa, J.M.; Manzano, R.; Carpena-Ruiz, R.O.; Gamarra, R.; Esteban, E. Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. J. Hazard Mater. 2009, 162, 854–859. [Google Scholar] [CrossRef]
- Pavlova, D.; Karadjova, I. Toxic Element Profiles in Selected Medicinal Plants Growing on Serpentines in Bulgaria. Biol. Trace Elem. Res. 2013, 156, 288–297. [Google Scholar] [CrossRef]
- Mihaljev, Z.; Zivkov-Balos, M.; Cupić, Z.; Jaksić, S. Levels of some microelements and essential heavy metals in herbal teas in Serbia. Acta Poloniae Pharm. 2014, 71, 385–391. [Google Scholar] [PubMed]
- Bonari, G.; Monaci, F.; Nannoni, F.; Angiolini, C.; Protano, G. Trace Element Uptake and Accumulation in the Medicinal Herb Hypericum perforatum L. Across Different Geolithological Settings. Biol. Trace Elem. Res. 2019, 189, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Obratov-Petković, D.; Bjedov, I.; Belanović, S. The content of heavy metals in the leaves of Hypericum perforatum L. on serpentinite soils in Serbia. Glas. Šumarskog Fak. 2008, 143–153. [Google Scholar] [CrossRef]
- Schneider, M.; Marquard, D.R. Investigations on the uptake of cadmium in Hypercum perforatum. L. (St. John’s wort). Int. Symp. Med. Ar. Plant 1995, 426, 435–442. [Google Scholar] [CrossRef]
- Lazzara, S. Ottimizzazione Delle Strategie di Conservazione del Germoplasma di Hypericum spp. della Flora Spontanea Siciliana. Ph.D. Thesis, Università degli Studi di Palermo, Palermo, Italy, 2015. [Google Scholar]
- Ullrich, S.M.; Ramsey, M.H.; Helios-Rybicka, E. Total and exchangeable concentrations of heavy metals in soils near Bytom, an area of Pb/Zn mining and smelting in Upper Silesia, Poland. J. Appl. Geochem. 1999, 14, 187–196. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 4 September 2020).
- Bargagli, R. Trace Elements in Terrestrial Plants; Springer: Berlin, Germany, 1998; p. 171. [Google Scholar]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Chugh, L.K.; Sawhney, S.K. Effect of cadmium on germination, amylases and rate of respiration of germinating pea seeds. Environ. Pollut. 1996, 92, 1–5. [Google Scholar] [CrossRef]
- Bishnoi, N.R.; Sheoran, I.S.; Singh, R. Effect of cadmium and nickel on mobilisation of food reserves and activities of hydrolytic enzymes in germinating pigeon pea seeds. Biol. Plant. 1993, 35, 583. [Google Scholar] [CrossRef]
- Kuriakose, S.V.; Prasad, M.N.V. Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul. 2008, 54, 143–156. [Google Scholar] [CrossRef]
- Moya, J.L.; Ros, R.; Picazo, I. Influence of cadmium and nickel on growth, net photosynthesis and carbohydrate distribution in rice plants. Photosynth. Res. 1993, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, S.; Rama, S.D. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Koeppe, D.E. The uptake, distribution, and effect of cadmium and lead in plants. Sci. Total Environ. 1977, 7, 197–206. [Google Scholar] [CrossRef]
- Malone, C.; Koeppe, D.E.; Miller, R.J. Localization of Lead Accumulated by Corn Plants. Plant Physiol. 1974, 53, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Burzyński, M.; Kłobus, G. Changes of photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress. Photosynthetica 2004, 42, 505–510. [Google Scholar] [CrossRef]
- Påhlsson, A.M. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water Air Soil Pollut. 1989, 47, 287–319. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Małecka, A.; Piechalak, A.; Morkunas, I.; Tomaszewska, B. Accumulation of lead in root cells of Pisum sativum. Acta Physiol. Plant. 2008, 30, 629–637. [Google Scholar] [CrossRef]
- Rotkittikhun, P.; Kruatrachue, M.; Chaiyarat, R.; Ngernsansaruay, C.; Pokethitiyook, P.; Paijitprapaporn, A.; Baker, A.J.M. Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ. Pollut. 2006, 144, 681–688. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Tung, G.; Temple, P.J. Uptake and localization of lead in corn (Zea mays L.) seedlings, a study by histochemical and electron microscopy. Sci. Total Environ. 1996, 188, 71–85. [Google Scholar] [CrossRef]
- Seregin, I.V.; Shpigun, L.K.; Ivanov, V.B. Distribution and Toxic Effects of Cadmium and Lead on Maize Roots. Russ. J. Plant Physiol. 2004, 51, 525–533. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Gasser, U.; Klier, B.; Kühn, A.V.; Steinhoff, B. Current Findings on the Heavy Metal Content in Herbal Drugs. Pharmeuropa 2009, 1, 37–49. [Google Scholar]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Ismat, N.; Hifsa, M.; Zeb, S. Characterization of heavy metals in extracts of Hypericum medicinal plant by flame atomic absorption spectrometry. Asian J. Chem. 2010, 22, 4387–4392. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials. Available online: https://apps.who.int/iris/handle/10665/41986 (accessed on 3 December 2020).
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Pinto, A.; Mota, A.; Devarennes, A.; Pinto, F. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 2004, 326, 239–247. [Google Scholar] [CrossRef]
- Babula, P.; Klejdus, B.; Kovacik, J.; Hedbavny, J.; Hlavna, M. Lanthanum rather than cadmium induces oxidative stress and metabolite changes in Hypericum perforatum. J. Hazard. Mater. 2015, 286, 334–342. [Google Scholar] [CrossRef]
- Parekh, D.; Puranik, R.M.; Srivastava, H.S. Inhibition of Chlorophyll Biosynthesis by Cadmium in Greening Maize Leaf Segments. Biochem. Physiol. Pflanz. 1990, 186, 239–242. [Google Scholar] [CrossRef]
- Ewais, E.A. Effects of cadmium, nickel and lead on growth, chlorophyll content and proteins of weeds. Biol. Plant. 1997, 39, 403–410. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Lagriffoul, A.; Mocquot, B.; Mench, M.; Vangronsveld, J. Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 1998, 200, 241–250. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.; Spiller, M. In Situ detection of heavy metal substituted chlorophylls in water plants. Photosynth. Res. 1998, 58, 123–133. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Spectral reflectance and leaf internal structure changes of barley plants due to phytoextraction of zinc and cadmium. Int. J. Remote Sens. 2007, 28, 1041–1054. [Google Scholar] [CrossRef]
- Godinho, D.P.; Serrano, H.C.; Da Silva, A.B.; Branquinho, C.; Magalhães, S. Effect of Cadmium Accumulation on the Performance of Plants and of Herbivores that Cope Differently with Organic Defenses. Front. Plant Sci. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Hadi, F.; Aziz, T. A mini review on lead (Pb) toxicity in plants. J. Biol. Life Sci. 2015, 6, 91–101. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Boil. 2000, 3, 211–216. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Physiological Changes in Nitrogen-and. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Fernandez, S.; Vidal, D.; Simon, E.; SOLl3-SUGRANES, L. Radiometric characteristics of Triticum aestivum cv, Astral under water and nitrogen stress. Int. J. Remote Sens. 1994, 15, 1867–1884. [Google Scholar] [CrossRef]
- Živčák, M.; Olšovská, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestič, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2015, 60, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Swoczyna, T.; Lata, B.; Stasiak, A.; Stefaniak, J.; Latocha, P. JIP-test in assessing sensitivity to nitrogen deficiency in two cultivars of Actinidia arguta (Siebold et Zucc.) Planch. ex Miq. Photosynthectica 2019, 57, 646–658. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Germination | Seedling Length |
|---|---|---|
| Control | 73.06 ± 3.27 a | 4.27 ± 0.26 a |
| Cd 10 µM | 81.06 ± 1.49 a | 4.71 ± 0.19 a |
| Cd 100 µM | 83.50 ± 3.40 a | 0.82 ± 0.25 b |
| Pb 10 µM | 66.11 ± 4.06 a | 4.42 ± 0.63 a |
| Pb 100 µM | 79.61 ± 4.96 a | 3.72 ± 0.52 a |
| Treatment | Chlorophyll (mg·m−2) | NDVI | FV/FM | PI |
|---|---|---|---|---|
| Control | 1.14 ± 0.05 a | 1.05 ± 0.04 ab | 0.99 ± 0.01 a | 0.83 ± 0.07 a |
| Cd 10 µM | 0.92 ± 0.02 b | 1.17 ± 0.12 ab | 0.99 ± 0.03 a | 0.92 ± 0.12 a |
| Cd 100 µM | 0.85 ± 0.03 b | 0.90 ± 0.03 a | 0.98 ± 0.01a | 0.84 ± 0.06 a |
| Pb 10 µM | 1.04 ± 0.02 a | 1.27 ± 0.09 b | 1.01 ± 0.004 a | 0.87 ± 0.05 a |
| Pb 100 µM | 1.04 ± 0.02 a | 1.26 ± 0.10 b | 1.00 ± 0.01 a | 1.03 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafarova, M.; Vannini, A.; Monaci, F.; Loppi, S. Influence of Moderate Cd and Pb Soil Pollution on Seed Development, Photosynthetic Performance and Foliar Accumulation in the Medicinal Plant Hypericum perforatum. Pollutants 2021, 1, 1-9. https://0-doi-org.brum.beds.ac.uk/10.3390/pollutants1010001
Jafarova M, Vannini A, Monaci F, Loppi S. Influence of Moderate Cd and Pb Soil Pollution on Seed Development, Photosynthetic Performance and Foliar Accumulation in the Medicinal Plant Hypericum perforatum. Pollutants. 2021; 1(1):1-9. https://0-doi-org.brum.beds.ac.uk/10.3390/pollutants1010001
Chicago/Turabian StyleJafarova, Mehriban, Andrea Vannini, Fabrizio Monaci, and Stefano Loppi. 2021. "Influence of Moderate Cd and Pb Soil Pollution on Seed Development, Photosynthetic Performance and Foliar Accumulation in the Medicinal Plant Hypericum perforatum" Pollutants 1, no. 1: 1-9. https://0-doi-org.brum.beds.ac.uk/10.3390/pollutants1010001






