Psychopathology and Neurocognition in the Era of the p-Factor: The Current Landscape and the Road Forward
Abstract
:1. Introduction
2. Classifying Psychopathology
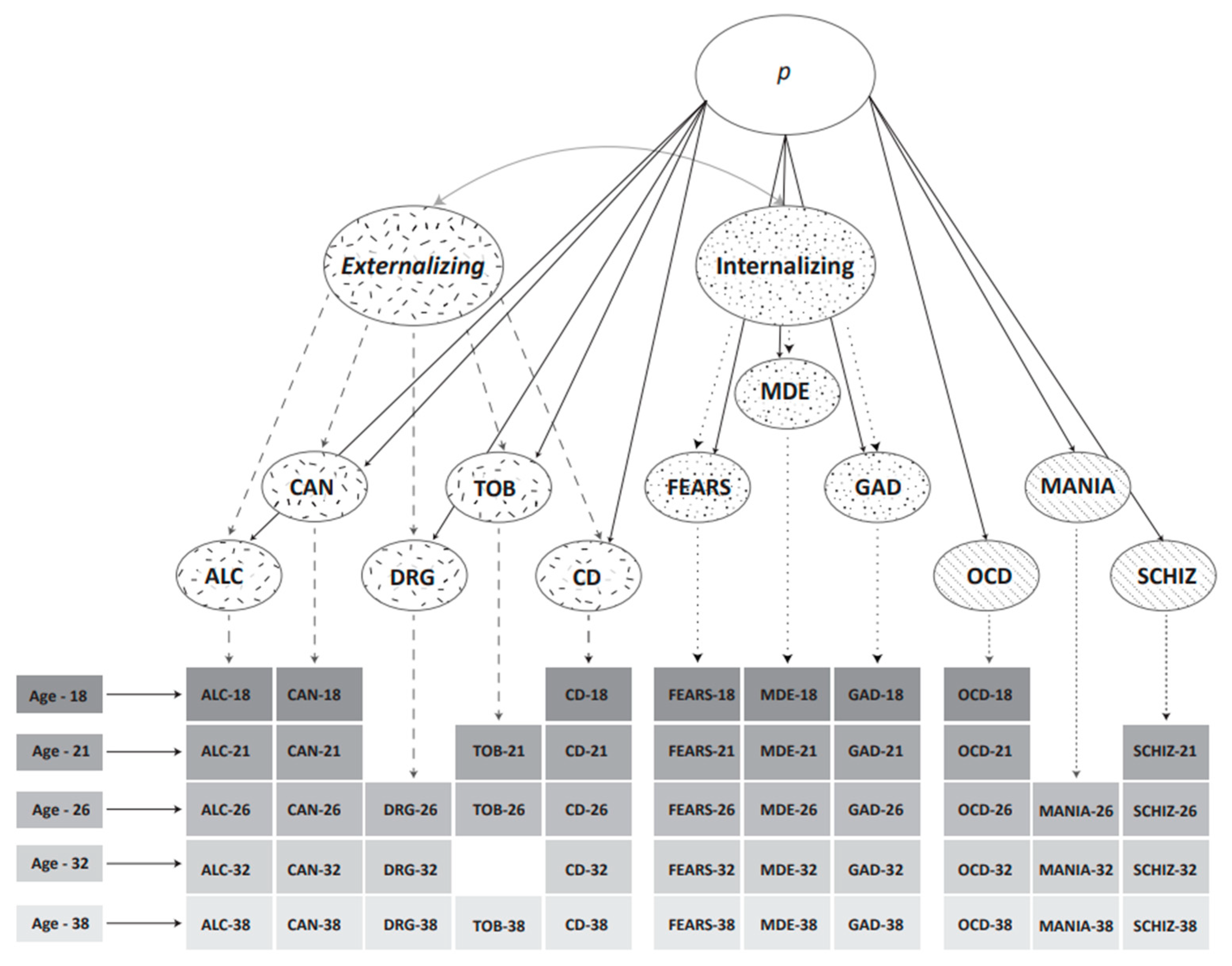
3. Caspi et al.’s Structural Models of Psychopathology
4. What Is the p-Factor?
4.1. Neurocognitive Abilities as Important to the Factors of Psychopathology
4.2. Deficits in Neurocognitive Processes and Their Relation to Psychopathology
4.2.1. Internalising
4.2.2. Externalising
4.2.3. Thought Disorder
5. A Mechanistic Approach
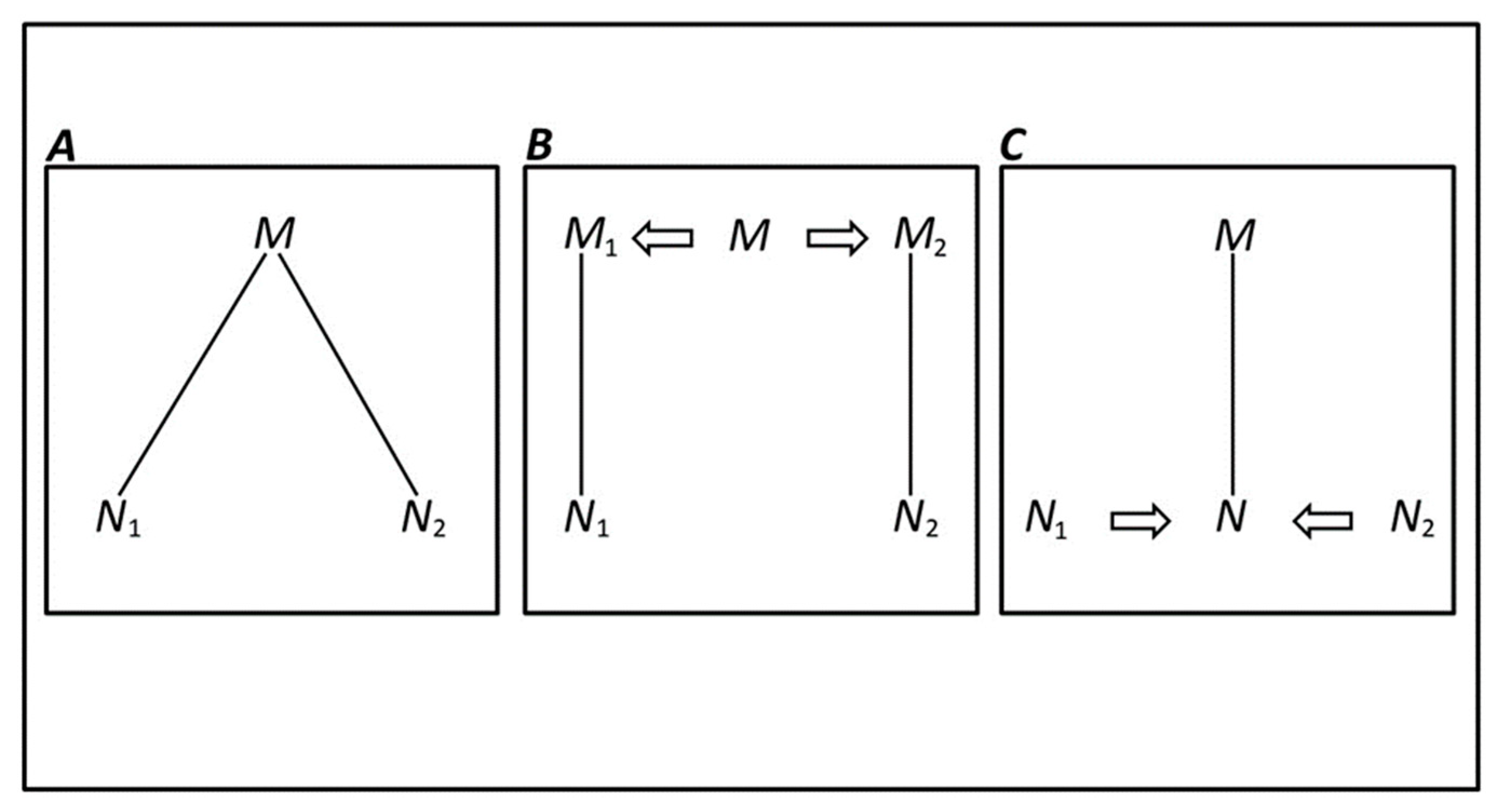
6. p, Substantive Factor, or Statistical Artifact?
An Alternative Appraoch
7. Heterogeneity of Psychopathology and Neurocognition
Multiple Realisation and Psychopathology
8. The Road Forward
- a.
- Further examine if a universal substantive p (and specific factors) could be developed the by assessment of the utility and consistency of structural models of psychopathology in subgroups.
- b.
- Utilise the S-1 bifactor model in explorations of neurocognitive ability and psychopathology.
- c.
- Assess if, at the population level, each of the factors of psychopathology are each best explained by a single or a small number of pattern(s) of neurocognitive component ability levels, with little variability.
- d.
- Assess if each factor of psychopathology (e.g., internalising, externalising and thought disorder) is usefully explained at the individual level by different combinations of ability levels of the components of neurocognition (e.g., the multidimensional hypothesis). This would support the proposition that neurocognition’s association to psychopathology, at a mechanistic level, is individual.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, H.R.; Hutchison, N.; Nyhus, E.; Curran, T.; Banich, M.T.; O'Reilly, R.C.; Munakata, Y. Neural inhibition enables selection during language processing. Proc. Natl. Acad. Sci. USA 2010, 107, 16483–16488. [Google Scholar] [CrossRef] [Green Version]
- Galletly, C.A.; MacFarlane, A.C.; Clark, C.R. Impaired updating of working memory in schizophrenia. Int. J. Psychophysiol. 2007, 63, 265–274. [Google Scholar] [CrossRef]
- Gilleen, J.; David, A.; Greenwood, K. Self-reflection and set-shifting mediate awareness in cognitively preserved schizophrenia patients. Cogn. Neuropsychiatry 2016, 21, 185–196. [Google Scholar] [CrossRef]
- Kiehl, K.A.; Smith, A.M.; Hare, R.D.; Liddle, P.F. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol. Psychiatry 2000, 48, 210–221. [Google Scholar] [CrossRef]
- De Lissnyder, E.; Koster, E.H.W.; Derakshan, N.; De Raedt, R. The association between depressive symptoms and executive control impairments in response to emotional and non-emotional information. Cogn. Emot. 2010, 24, 264–280. [Google Scholar] [CrossRef] [Green Version]
- Joormann, J.; Yoon, K.L.; Zetsche, U. Cognitive inhibition in depression. Appl. Prev. Psychol 2007, 12, 128–139. [Google Scholar] [CrossRef]
- Joormann, J.; Gotlib, I.H. Updating the contents of working memory in depression: Interference from irrelevant negative material. J. Abnorm. Psychol. 2008, 117, 182. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.J.; Funk, S.G.; Young, S.Y.; Schiöth, H.B. The role of working memory for cognitive control in anorexia nervosa versus substance use disorder. Front. Psychol. 2017, 8, 1651. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, O.M.; Goldenberg, D.; Thayer, R.; Migliorini, R.; Simmons, A.N.; Tapert, S.F. Adolescents' fMRI activation to a response inhibition task predicts future substance use. Addict. Behav. 2013, 38, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Noël, X.; Brevers, D.; Bechara, A. A neurocognitive approach to understanding the neurobiology of addiction. Curr. Opin. Neurobiol. 2013, 23, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.L.; Moffitt, T.E.; Caspi, A.; Silva, P.A. Comorbid mental disorders: Implications for treatment and sample selection. J. Abnorm. Psychol. 1998, 107, 305. [Google Scholar] [CrossRef]
- Kotov, R.; Krueger, R.F.; Watson, D.; Achenbach, T.M.; Althoff, R.R.; Bagby, R.M.; Brown, T.A.; Carpenter, W.T.; Caspi, A.; Clark, L.A. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 2017, 126, 454. [Google Scholar] [CrossRef] [Green Version]
- Caspi, A.; Houts, R.M.; Belsky, D.W.; Goldman-Mellor, S.J.; Harrington, H.; Israel, S.; Meier, M.H.; Ramrakha, S.; Shalev, I.; Poulton, R. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014, 2, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Lahey, B.B.; Applegate, B.; Hakes, J.K.; Zald, D.H.; Hariri, A.R.; Rathouz, P.J. Is there a general factor of prevalent psychopathology during adulthood? J. Abnorm. Psychol. 2012, 121, 971. [Google Scholar] [CrossRef] [Green Version]
- Aldao, A.; Gee, D.G.; De Los Reyes, A.; Seager, I. Emotion regulation as a transdiagnostic factor in the development of internalizing and externalizing psychopathology: Current and future directions. Dev. Psychopathol. 2016, 28, 927–946. [Google Scholar] [CrossRef]
- McManus, F.; Shafran, R.; Cooper, Z. What does a transdiagnostic approach have to offer the treatment of anxiety disorders? Br. J. Clin. Psychol. 2010, 49, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Mansell, W.; Carey, T.A.; Tai, S. A Transdiagnostic Approach to CBT Using Method of Levels Therapy: Distinctive Features; Routledge: Oxfordshire, UK, 2012. [Google Scholar]
- Haywood, D.; Baughman, F.D. Multidimensionality in Executive Function Profiles in Schizophrenia: A Computational Approach Using the Wisconsin Card Sorting Task. Comput. Brain Behav. 2021, 1–14. [Google Scholar] [CrossRef]
- Rybakowski, J.K. 120th anniversary of the Kraepelinian dichotomy of psychiatric disorders. Curr. Psychiatry Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Krueger, R.F.; Eaton, N.R. Transdiagnostic factors of mental disorders. World Psychiatry 2015, 14, 27–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.A.; Watson, D.; Reynolds, S. Diagnosis and classification of psychopathology: Challenges to the current system and future directions. Ann. Rev. Psychol. 1995, 46, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Hovenkamp-Hermelink, J.H.M.; Riese, H.; Batelaan, N.M.; Penninx, B.W.J.H.; Schoevers, R.A. Low stability of diagnostic classifications of anxiety disorders over time: A six-year follow-up of the NESDA study. J. Affect. Disord. 2016, 190, 310–315. [Google Scholar] [CrossRef]
- Craddock, N.; Owen, M.J. The Kraepelinian dichotomy–going, going… but still not gone. Br. J. Psychiatry 2010, 196, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Yip, B.H.; Björk, C.; Pawitan, Y.; Cannon, T.D.; Sullivan, P.F.; Hultman, C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet 2009, 373, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Smucny, J.; Lesh, T.A.; Newton, K.; Niendam, T.A.; Ragland, J.D.; Carter, C.S. Levels of cognitive control: A functional magnetic resonance imaging-based test of an RDoC domain across bipolar disorder and schizophrenia. Neuropsychopharmacology 2018, 43, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Burdick, K.E.; Goldberg, J.F.; Harrow, M.; Faull, R.N.; Malhotra, A.K. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J. Nerv. Ment. Dis. 2006, 194, 255–260. [Google Scholar] [CrossRef]
- Kotov, R.; Krueger, R.F.; Watson, D.; Cicero, D.C.; Conway, C.C.; DeYoung, C.G.; Eaton, N.R.; Forbes, M.K.; Hallquist, M.N.; Latzman, R.D. The Hierarchical Taxonomy of Psychopathology (HiTOP): A Quantitative Nosology Based on Consensus of Evidence. Annu. Rev. Clin. Psychol. 2021, 17, 83–108. [Google Scholar] [CrossRef]
- Robins, L.N.; Helzer, J.E.; Croughan, J.; Ratcliff, K.S. National Institute of Mental Health diagnostic interview schedule: Its history, characteristics, and validity. Arch. Gen. Psychiatry 1981, 38, 381–389. [Google Scholar] [CrossRef]
- Watts, A.L.; Lane, S.P.; Bonifay, W.; Steinley, D.; Meyer, F. Building theories on top of, and not independent of, statistical models: The case of the p-factor. Psychol. Inq. 2020, 31, 310–320. [Google Scholar] [CrossRef]
- Brandes, C.M.; Herzhoff, K.; Smack, A.J.; Tackett, J.L. The p factor and the n factor: Associations between the general factors of psychopathology and neuroticism in children. Clin. Psychol. Sci. 2019, 7, 1266–1284. [Google Scholar] [CrossRef]
- Smith, G.T.; Atkinson, E.A.; Davis, H.A.; Riley, E.N.; Oltmanns, J.R. The general factor of psychopathology. Annu. Rev. Clin. Psychol. 2020, 16, 75–98. [Google Scholar] [CrossRef] [Green Version]
- Carver, C.S.; Johnson, S.L.; Timpano, K.R. Toward a functional view of the p factor in psychopathology. Clin. Psychol. Sci. 2017, 5, 880–889. [Google Scholar] [CrossRef]
- Caspi, A.; Moffitt, T.E. All for one and one for all: Mental disorders in one dimension. Am. J. Psychiatry 2018, 175, 831–844. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.; Geiser, C.; Zagorscak, P.; Burns, G.L.; Bohn, J.; Becker, S.P.; Eid, M.; Beauchaine, T.P.; Knaevelsrud, C. On the meaning of the general factor of psychopathology (“P-Factor”) in symmetrical bifactor models. IpsyArxiv 2020. [Google Scholar] [CrossRef]
- Crow, A.J.D. Associations between neuroticism and executive function outcomes: Response inhibition and sustained attention on a Continuous Performance Test. Percept. Mot. Ski. 2019, 126, 623–638. [Google Scholar] [CrossRef]
- Lynch, S.J.; Sunderland, M.; Newton, N.C.; Chapman, C. A systematic review of transdiagnostic risk and protective factors for general and specific psychopathology in young people. Clin. Psychol. Rev. 2021, 102036. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Fischer, J.S. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Norman, D.A.; Shallice, T. Attention to action. In Consciousness and Self-Regulation; Springer: New York, NY, USA, 1986; pp. 1–18. [Google Scholar]
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Carter, A.; Reznick, J.S.; Frye, D. Early development of executive function: A problem-solving framework. Rev. Gen. Psychol. 1997, 1, 198–226. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salthouse, T.A. The processing-speed theory of adult age differences in cognition. Psychol. Rev. 1996, 103, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.R. Emotional attention set-shifting and its relationship to anxiety and emotion regulation. Emotion 2009, 9, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsourtos, G.; Thompson, J.C.; Stough, C. Evidence of an early information processing speed deficit in unipolar major depression. Psychol. Med. 2002, 32, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Endres, M.J.; Rickert, M.E.; Bogg, T.; Lucas, J.; Finn, P.R. Externalizing psychopathology and behavioral disinhibition: Working memory mediates signal discriminability and reinforcement moderates response bias in approach–avoidance learning. J. Abnorm. Psychol. 2011, 120, 336. [Google Scholar] [CrossRef] [Green Version]
- Endres, M.J.; Donkin, C.; Finn, P.R. An information processing/associative learning account of behavioral disinhibition in externalizing psychopathology. Exp. Clin. Psychopharmacol. 2014, 22, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, L.A.; Ryan, M.; Martin, R.B.; Ewen, J.; Mostofsky, S.H.; Denckla, M.B.; Mahone, E.M. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychol. 2011, 17, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, M.A.; Pennington, B.F.; Yerys, B.E.; Scott, A.; Boada, R.; Willcutt, E.G.; Olson, R.K.; DeFries, J.C. Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. J. Abnorm. Child Psychol. 2006, 34, 584. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, M.M.; Gerraty, R.T. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology 2009, 23, 551. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, J.M.; Crespo-Facorro, B.; González-Blanch, C.; Perez-Iglesias, R.; Vázquez-Barquero, J.L. Cognitive dysfunction in first-episode psychosis: The processing speed hypothesis. Br. J. Psychiatry 2007, 191, s107–s110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbert, B.N. The role of RDoC in future classification of mental disorders. Dialogues Clin. Neurosci. 2020, 22, 81. [Google Scholar] [PubMed]
- Cuthbert, B.N.; Kozak, M.J. Constructing constructs for psychopathology: The NIMH research domain criteria. J. Abnorm. Psychol. 2013, 122, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, T.; Kang, E.; Capriola-Hall, N.; Lerner, M.D.; Jarcho, J.; Prinstein, M.J. Meta-Analysis of the RDoC Social Processing Domain across Units of Analysis in Children and Adolescents. J. Clin. Child Adolesc. Psychol. 2019, 9, 1–25. [Google Scholar] [CrossRef]
- Ip, K.I.; Jester, J.M.; Sameroff, A.; Olson, S.L. Linking Research Domain Criteria (RDoC) constructs to developmental psychopathology: The role of self-regulation and emotion knowledge in the development of internalizing and externalizing growth trajectories from ages 3 to 10. Dev. Psychopathol. 2019, 31, 1557–1574. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.J.; Venables, N.C.; Yancey, J.R.; Hicks, B.M.; Nelson, L.D.; Kramer, M.D. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. J. Abnorm. Psychol. 2013, 122, 902. [Google Scholar] [CrossRef]
- Snyder, H.R.; Hankin, B.L. All models are wrong, but the p factor model is useful: Reply to Widiger and Oltmanns (2017) and Bonifay, Lane, and Reise (2017). Clin. Psychol. Sci. 2017, 5, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahey, B.B.; Moore, T.M.; Kaczkurkin, A.N.; Zald, D.H. Hierarchical models of psychopathology: Empirical support, implications, and remaining issues. World Psychiatry 2021, 20, 57–63. [Google Scholar] [CrossRef]
- Levin-Aspenson, H.F.; Watson, D.; Clark, L.A.; Zimmerman, M. What is the general factor of psychopathology? Consistency of the p factor across samples. Assessment 2020, 28, 1035–1049. [Google Scholar] [CrossRef]
- Kessler, R.C.; Merikangas, K.R. The national comorbidity survey replication (NCS-R): Background and aims. Int. J. Methods Psychiatr. Res. 2004, 13, 60–68. [Google Scholar] [CrossRef]
- Heeringa, S.G.; Wagner, J.; Torres, M.; Duan, N.; Adams, T.; Berglund, P. Sample designs and sampling methods for the Collaborative Psychiatric Epidemiology Studies (CPES). Int. J. Methods Psychiatr. Res. 2004, 13, 221–240. [Google Scholar] [CrossRef]
- Zimmerman, M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can. J. Psychiatry 2016, 61, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Greene, A.L.; Eaton, N.R.; Li, K.; Forbes, M.K.; Krueger, R.F.; Markon, K.E.; Waldman, I.D.; Cicero, D.C.; Conway, C.C.; Docherty, A.R. Are fit indices used to test psychopathology structure biased? A simulation study. J. Abnorm. Psychol. 2019, 128, 740. [Google Scholar] [CrossRef] [PubMed]
- Fried, E.I.; Greene, A.L.; Eaton, N.R. The p factor is the sum of its parts, for now. World Psychiatry 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Haywood, D.; Baughman, F.; Mullan, B.; Heslop, K.R. Going “Up” to Move Forward: S-1 Bifactor Models and the Study of Neurocognitive Abilities in Psychopathology. PsyArxiv 2021. [Google Scholar] [CrossRef]
- Eid, M. Multi-faceted constructs in abnormal psychology: Implications of the bifactor S-1 model for individual clinical assessment. J. Abnorm. Child Psychol. 2020, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Burke, J.D.; Johnston, O.G. The bifactor S-1 model: A psychometrically sounder alternative to test the structure of ADHD and ODD? J. Abnorm. Child Psychol. 2020, 48, 911–915. [Google Scholar] [CrossRef]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.-H.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef] [Green Version]
- International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009, 460, 748. [Google Scholar] [CrossRef]
- Cowen, P.J. Neuroendocrine and neurochemical processes in depression. Psychopathol. Rev. 2016, 3, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Stefanopoulou, E.; Manoharan, A.; Landau, S.; Geddes, J.R.; Goodwin, G.U.Y.; Frangou, S. Cognitive functioning in patients with affective disorders and schizophrenia: A meta-analysis. Int. Rev. Psychiatry 2009, 21, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Szöke, A.; Schürhoff, F.; Mathieu, F.; Meary, A.; Ionescu, S.; Leboyer, M. Tests of executive functions in first-degree relatives of schizophrenic patients: A meta-analysis. Psychol. Med. 2005, 35, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Barceló, F.; Knight, R.T. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia 2002, 40, 349–356. [Google Scholar] [CrossRef]
- Hartman, M.; Bolton, E.; Fehnel, S.E. Accounting for age differences on the Wisconsin Card Sorting Test: Decreased working memory, not inflexibility. Psychol. Aging 2001, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Manoach, D.S.; Lindgren, K.A.; Cherkasova, M.V.; Goff, D.C.; Halpern, E.F.; Intriligator, J.; Barton, J.J.S. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol. Psychiatry 2002, 51, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Gamboz, N.; Borella, E.; Brandimonte, M.A. The role of switching, inhibition and working memory in older adults’ performance in the Wisconsin Card Sorting Test. Aging Neuropsychol. Cogn. 2009, 16, 260–284. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.J.; Strejilevich, S.A.; Scápola, M.; Igoa, A.; Marengo, E.; Ais, E.D.; Perinot, L. Heterogeneity in cognitive functioning among patients with bipolar disorder. J. Affect. Disord. 2008, 109, 149–156. [Google Scholar] [CrossRef]
- Raffard, S.; Bayard, S. Understanding the executive functioning heterogeneity in schizophrenia. Brain Cogn. 2012, 79, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Birkner, C.; Kloss, M.; Jahn, H.; Hand, I.; Haasen, C.; Krausz, M. Executive functioning in obsessive–compulsive disorder, unipolar depression, and schizophrenia. Arch. Clin. Neuropsychol. 2002, 17, 477–483. [Google Scholar] [PubMed]
- Bringmann, L.F.; Eronen, M.I. Don’t blame the model: Reconsidering the network approach to psychopathology. Psychol. Rev. 2018, 125, 606. [Google Scholar] [CrossRef] [Green Version]
- Putnam, H. Representation and Reality; MIT Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Pavão, R.; Tort, A.B.L.; Amaral, O.B. Multifactoriality in psychiatric disorders: A computational study of schizophrenia. Schizophr. Bull. 2015, 41, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Bechtel, W.; Mundale, J. Multiple realizability revisited: Linking cognitive and neural states. Philos. Sci. 1999, 66, 175–207. [Google Scholar] [CrossRef]
- Bregant, J. John Bickle, Philosophy and Neuroscience: A Ruthlessly Reductive Account. Croat. J. Philos. 2006, 6, 133–140. [Google Scholar]
- Polger, T.W.; Shapiro, L.A. The Multiple Realization Book; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Pernu, T.K. Elimination, not reduction: Lessons from the Research Domain Criteria (RDoC) and multiple realisation. Behav. Brain Sci. 2019, 42, 22. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L. Neural reuse: A fundamental organizational principle of the brain. Behav. Brain Sci. 2010, 33, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-C.; Kim, J.-M.; Jun, T.-Y.; Lee, M.-S.; Kim, J.-B.; Yim, H.-W.; Park, Y.C. How many different symptom combinations fulfil the diagnostic criteria for major depressive disorder? Results from the CRESCEND study. Nord. J. Psychiatry 2017, 71, 217–222. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haywood, D.; Baughman, F.D.; Mullan, B.A.; Heslop, K.R. Psychopathology and Neurocognition in the Era of the p-Factor: The Current Landscape and the Road Forward. Psychiatry Int. 2021, 2, 233-249. https://0-doi-org.brum.beds.ac.uk/10.3390/psychiatryint2030018
Haywood D, Baughman FD, Mullan BA, Heslop KR. Psychopathology and Neurocognition in the Era of the p-Factor: The Current Landscape and the Road Forward. Psychiatry International. 2021; 2(3):233-249. https://0-doi-org.brum.beds.ac.uk/10.3390/psychiatryint2030018
Chicago/Turabian StyleHaywood, Darren, Frank D. Baughman, Barbara A. Mullan, and Karen R. Heslop. 2021. "Psychopathology and Neurocognition in the Era of the p-Factor: The Current Landscape and the Road Forward" Psychiatry International 2, no. 3: 233-249. https://0-doi-org.brum.beds.ac.uk/10.3390/psychiatryint2030018





