Polyethylene Oxide Hydrogels Crosslinked by Peroxide for the Controlled Release of Proteins
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Hydrogel Synthesis and Preparation
2.3. Fourier Transform Infrared Spectroscopy
2.4. Swelling Measurements
2.5. Small Angle Neutron Scattering
2.6. Rheometry
2.7. Loading and Release of Proteins
2.8. UV-Vis Absorption Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwall, C.T.; Banerjee, I.A. Micro- and Nanoscale Hydrogel Systems for Drug Delivery and Tissue Engineering. Materials 2009, 2, 577–612. [Google Scholar] [CrossRef] [Green Version]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term Controlled Protein Release from Poly(Ethylene Glycol) Hydrogels by Modulating Mesh Size and Degradation. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zia, F.; Anjum, M.N.; Saif, M.J.; Jamil, T.; Malik, K.; Anjum, S.; BiBi, I.; Zia, M.A. Chapter 16–Alginate-Poly(Ethylene) Glycol and Poly(Ethylene) Oxide Blend Materials. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 581–601. [Google Scholar] [CrossRef]
- Whitehead, A.K.; Barnett, H.H.; Caldorera-Moore, M.E.; Newman, J.J. Poly(ethylene glycol) hydrogel elasticity influences human mesenchymal stem cell behavior. Regen. Biomater. 2018, 5, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Fu, R.-H.; Liao, S.-F.; Liu, S.-P.; Lin, S.-Z.; Wang, Y.-C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transpl. 2018, 27, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kiick, K.L. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules 2005, 6, 1921–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Bang, S.; Kim, S.; Jo, S.Y.; Kim, B.-C.; Hwang, Y.; Noh, I. Synthesis and in vitro characterizations of porous carboxymethyl cellulose-poly(ethylene oxide) hydrogel film. Biomater. Res. 2015, 19, 12. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; McDonald, A.G. Peroxide induced cross-linking by reactive melt processing of two biopolyesters: Poly(3-hydroxybutyrate) and poly(l-lactic acid) to improve their melting processability. J. Appl. Polym. Sci. 2015, 132, 41724. [Google Scholar] [CrossRef]
- Takamura, M.; Nakamura, T.; Kawaguchi, S.; Takahashi, T.; Koyama, K. Molecular characterization and crystallization behavior of peroxide-induced slightly crosslinked poly(L-lactide) during extrusion. Polym. J. 2010, 42, 600–608. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Gong, W.-G.; Zheng, B.-C. The Effect of Peroxide Cross-Linking on the Properties of Low-Density Polyethylene. J. Macromol. Sci. Part B 2014, 53, 67–77. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, J.; Huang, W.; Zhang, D.; Chen, J.; Yang, H.; Xue, X.; Jiang, B. Radical polymerization in the presence of a peroxide monomer: An approach to branched vinyl polymers. Polym. Chem. 2017, 8, 4428–4439. [Google Scholar] [CrossRef]
- Emami, S.H.; Salovey, R.; Hogen-Esch, T.E. Peroxide-mediated crosslinking of poly(ethylene oxide). J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3021–3026. [Google Scholar] [CrossRef]
- Emami, S.H.; Salovey, R.; Hogen-Esch, T.E. Degradable poly(ethylene oxide) hydrogels formed by crosslinking with tert-butylperoxybenzoate. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 520–527. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Adv. Funct. Mater. 2009, 19, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Zustiak, S.P.; Leach, J.B. Characterization of protein release from hydrolytically degradable poly(ethylene glycol) hydrogels. Biotechnol. Bioeng. 2011, 108, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.G.; Pedersen, J.S. Instrumental Smearing Effects in Radially Symmetric Small-Angle Neutron Scattering by Numerical and Analytical Methods. J. Appl. Crystallogr. 1995, 28, 105–114. [Google Scholar] [CrossRef]
- Radulescu, A.; Szekely, N.K.; Polachowski, S.; Leyendecker, M.; Amann, M.; Buitenhuis, J.; Drochner, M.; Engels, R.; Hanslik, R.; Kemmerling, G.; et al. Tuning the instrument resolution using chopper and time of flight at the small-angle neutron scattering diffractometer KWS-2. J. Appl. Crystallogr. 2015, 48, 1849–1859. [Google Scholar] [CrossRef]
- Vad, T.; Sager, W.F.C.; Zhang, J.; Buitenhuis, J.; Radulescu, A. Experimental determination of resolution function parameters from small-angle neutron scattering data of a colloidal SiO2 dispersion. J. Appl. Crystallogr. 2010, 43, 686–692. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Horkay, F.; McKenna, G.B.; Deschamps, P.; Geissler, E. Neutron Scattering Properties of Randomly Cross-Linked Polyisoprene Gels. Macromolecules 2000, 33, 5215–5220. [Google Scholar] [CrossRef]
- Panyukov, S.; Rabin, Y. Polymer Gels: Frozen Inhomogeneities and Density Fluctuations. Macromolecules 1996, 29, 7960–7975. [Google Scholar] [CrossRef]
- Shibayama, M. Small-angle neutron scattering on polymer gels: Phase behavior, inhomogeneities and deformation mechanisms. Polym. J. 2011, 43, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Shibayama, M.; Kurokawa, H.; Nomura, S.; Muthukumar, M.; Stein, R.S.; Roy, S. Small-angle neutron scattering from poly(vinyl alcohol)-borate gels. Polymer 1992, 33, 2883–2890. [Google Scholar] [CrossRef]
- Papagiannopoulos, A. Chapter 10—Small-Angle Neutron Scattering (SANS). In Microscopy Methods in Nanomaterials Characterization; Thomas, S., Thomas, R., Zachariah, A.K., Mishra, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 339–361. [Google Scholar] [CrossRef]
- Saffer, E.M.; Lackey, M.A.; Griffin, D.M.; Kishore, S.; Tew, G.N.; Bhatia, S.R. SANS study of highly resilient poly(ethylene glycol) hydrogels. Soft Matter. 2014, 10, 1905–1916. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Rieker, T.; Agamalian, M.; Lin, J.S.; Fischer, D.; Sukumaran, S.; Chen, C.; Beaucage, G.; Herd, C.; Ivie, J. Multilevel structure of reinforcing silica and carbon. J. Appl. Crystallogr. 2000, 33, 587–591. [Google Scholar] [CrossRef]
- Schmidt, P.W. Small-angle scattering studies of disordered, porous and fractal systems. J. Appl. Crystallogr. 1991, 24, 414–435. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Rehmann, M.S.; Skeens, K.M.; Kharkar, P.M.; Ford, E.M.; Maverakis, E.; Lee, K.H.; Kloxin, A.M. Tuning and Predicting Mesh Size and Protein Release from Step Growth Hydrogels. Biomacromolecules 2017, 18, 3131–3142. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Hadjiev, N.A.; Amsden, B.G. An assessment of the ability of the obstruction-scaling model to estimate solute diffusion coefficients in hydrogels. J. Control. Release 2015, 199, 10–16. [Google Scholar] [CrossRef]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A 2009, 90A, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]

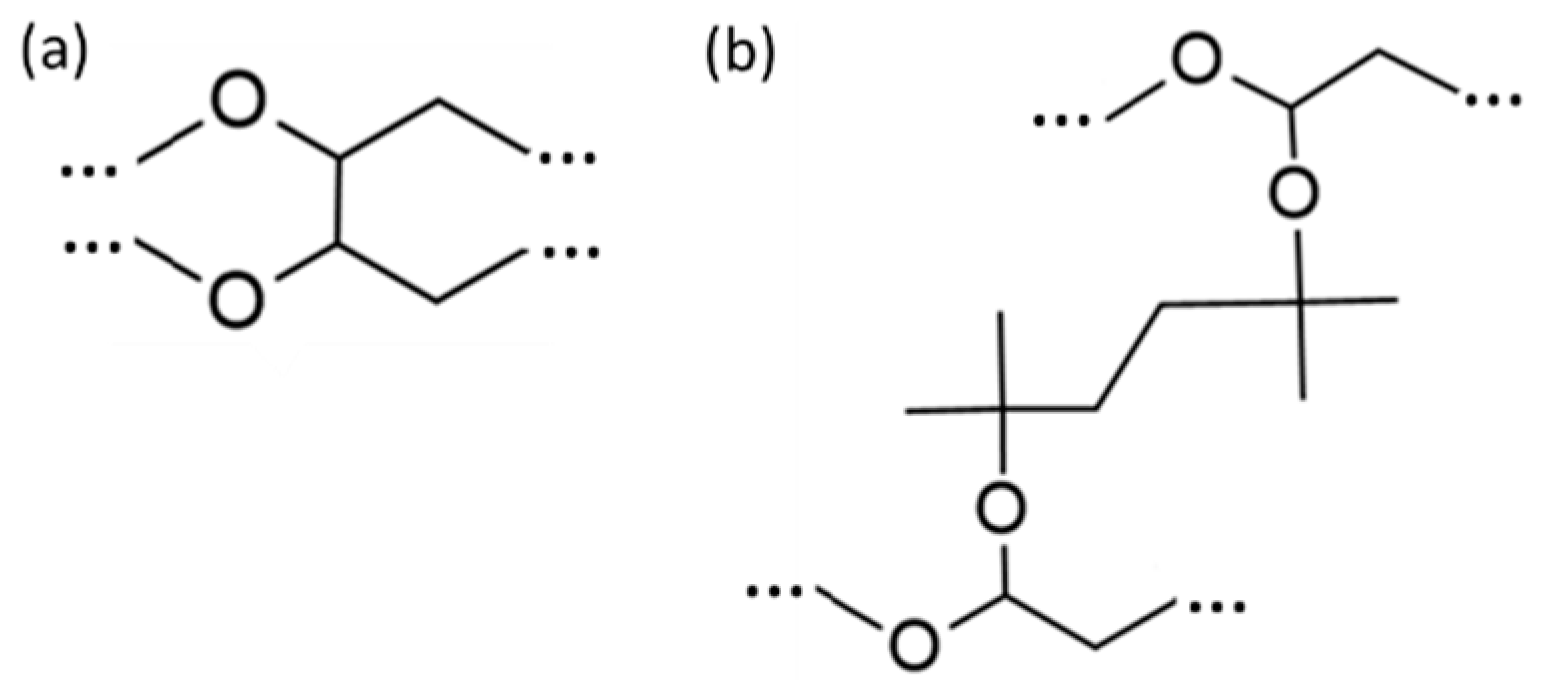
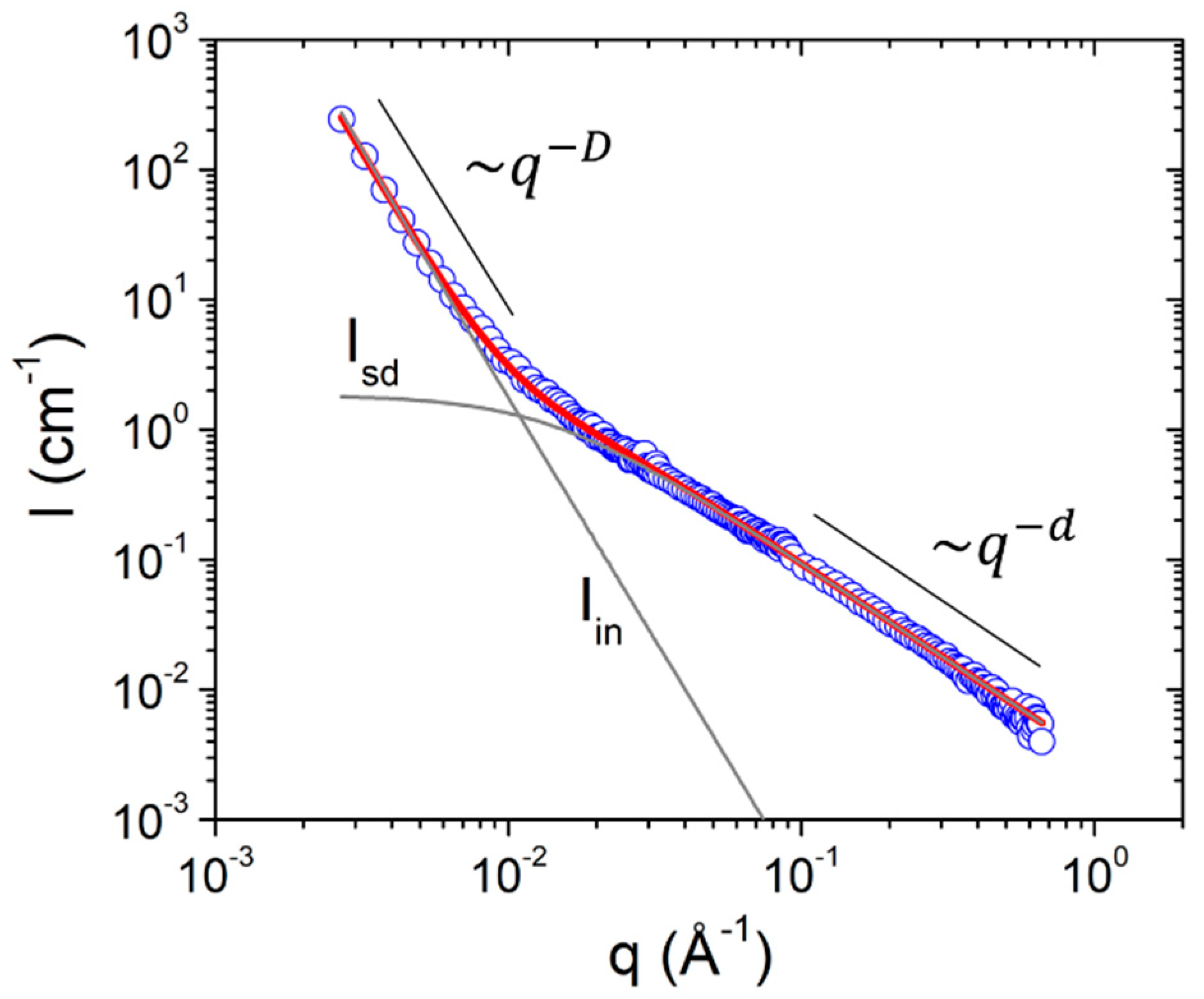
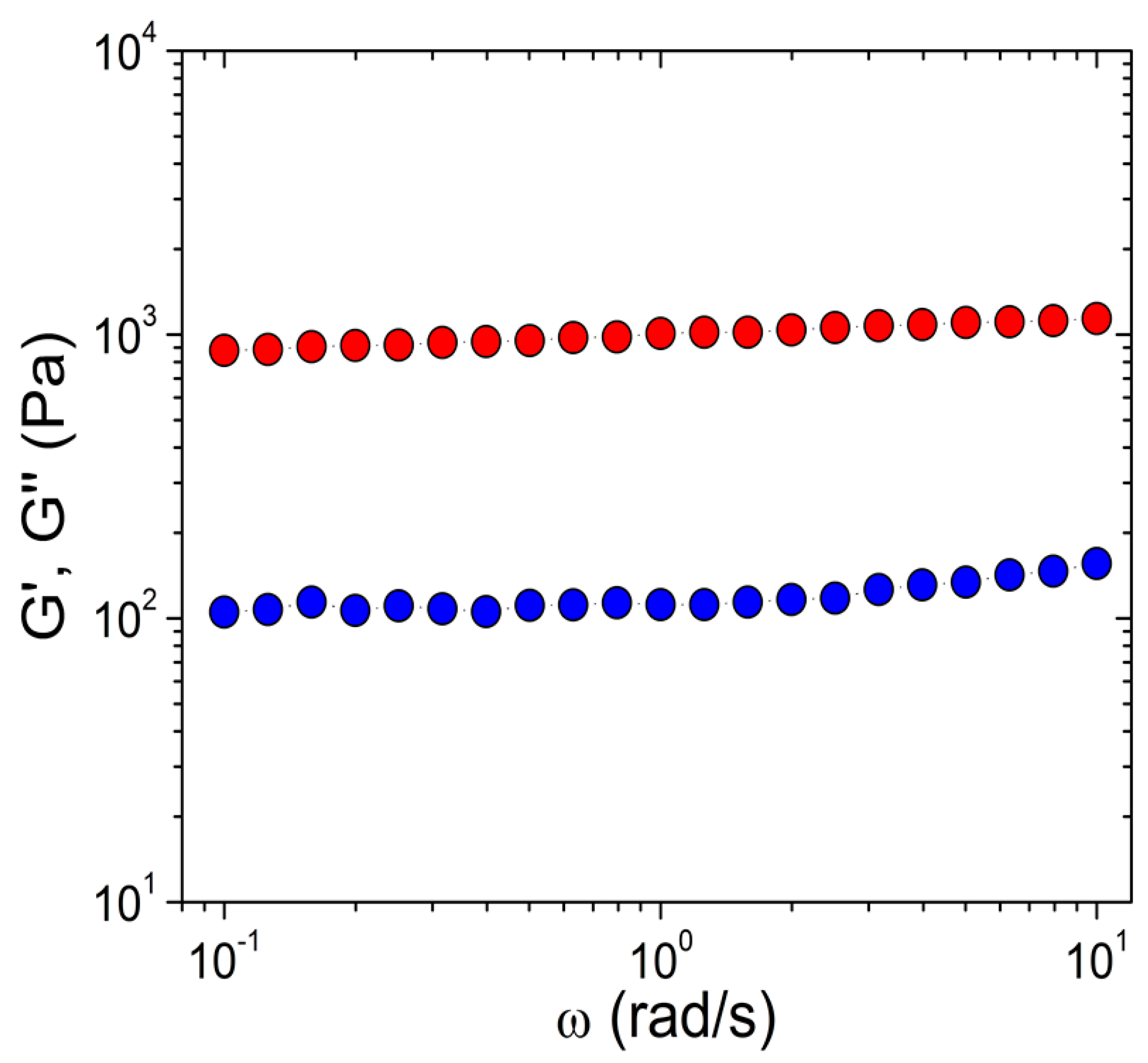

| Sample | % v/v Peroxide | Solvent |
|---|---|---|
| AC1 | 15 | Acetone |
| AC2 | 30 | |
| AC3 | 45 | |
| BUT1 | 15 | Butanol |
| BUT2 | 30 | |
| BUT3 | 45 | |
| DMF1 | 15 | Dimethyl-formamide |
| DMF2 | 30 | |
| DMF3 | 45 |
| Parameter/Sample | rs | ξcalc (nm) | G′calc (Pa) | ρ (1023 m−3) |
|---|---|---|---|---|
| AC1 | 635 ± 52 | 8.70 | 573 | 1.42 |
| AC2 | 605 ± 45 | 8.19 | 628 | 1.55 |
| AC3 | 541 ± 65 | 7.12 | 780 | 1.93 |
| BUT1 | 575 ± 63 | 7.68 | 693 | 1.71 |
| BUT2 | 548 ± 51 | 7.24 | 760 | 1.88 |
| BUT3 | 491 ± 41 | 6.32 | 938 | 2.32 |
| DMF1 | 551 ± 57 | 7.29 | 752 | 1.86 |
| DMF2 | 556 ± 56 | 7.37 | 739 | 1.83 |
| DMF3 | 423 ± 37 | 5.25 | 1250 | 3.09 |
| Parameter/Sample | A (10−8 cm−1 Å−D) | D | B (cm−1) | ξ (nm) | d |
|---|---|---|---|---|---|
| AC1 | 12.97 | 3.67 | 4.73 | 10.2 | 1.69 |
| AC2 | 45.76 | 3.46 | 2.38 | 6.78 | 1.57 |
| AC3 | 9.39 | 3.80 | 1.25 | 8.05 | 1.18 |
| BUT1 | 4.16 | 3.82 | 4.34 | 11.9 | 1.59 |
| BUT2 | 58.75 | 3.52 | 2.01 | 6.38 | 1.29 |
| BUT3 | 10.78 | 3.71 | 1.59 | 7.42 | 1.35 |
| DMF1 | 5.57 | 3.81 | 4.18 | 12.3 | 1.52 |
| DMF2 | 6.32 | 3.72 | 1.85 | 8.00 | 1.50 |
| DMF3 | 4.75 | 3.84 | 1.42 | 7.89 | 1.25 |
| % δP/P * | 3.1 | 3.5 | 9.8 | 8.7 | 2.0 |
| Parameter/Sample | G′ (Pa) | G″ (Pa) | tan δ |
|---|---|---|---|
| AC1 | 506 | 65.2 | 0.129 |
| AC2 | 825 | 74.2 | 0.090 |
| AC3 | 1003 | 119 | 0.119 |
| BUT1 | 695 | 52.0 | 0.075 |
| BUT2 | 725 | 13.4 | 0.018 |
| BUT3 | 959 | 123 | 0.128 |
| DMF1 | 251 | 27.6 | 0.110 |
| DMF2 | 902 | 38.1 | 0.042 |
| DMF3 | 1987 | 170 | 0.086 |
| Protein/Sample | BSA | LYZ |
|---|---|---|
| AC1 | 7.7 ± 0.8 | 36 ± 6 |
| AC3 | 5.2 ± 0.7 | 24 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagiannopoulos, A.; Vlassi, E.; Pispas, S.; Tsitsilianis, C.; Radulescu, A. Polyethylene Oxide Hydrogels Crosslinked by Peroxide for the Controlled Release of Proteins. Macromol 2021, 1, 37-48. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1010004
Papagiannopoulos A, Vlassi E, Pispas S, Tsitsilianis C, Radulescu A. Polyethylene Oxide Hydrogels Crosslinked by Peroxide for the Controlled Release of Proteins. Macromol. 2021; 1(1):37-48. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1010004
Chicago/Turabian StylePapagiannopoulos, Aristeidis, Eleni Vlassi, Stergios Pispas, Constantinos Tsitsilianis, and Aurel Radulescu. 2021. "Polyethylene Oxide Hydrogels Crosslinked by Peroxide for the Controlled Release of Proteins" Macromol 1, no. 1: 37-48. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1010004








