Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview
Abstract
:1. Introduction
2. Covalent Route for CNT Functionalization
2.1. “Grafting To” Strategy
2.1.1. Coupling and Nucleophilic Addition Reactions
2.1.2. Cycloaddition Reactions
2.1.3. Amide or Ester Linkage
2.1.4. Other “Grafting To” Approaches
2.2. “Grafting From” Strategy
2.2.1. Atom Transfer Radical Polymerization (ATRP)
2.2.2. Reversible Addition–Fragmentation Chain Transfer (RAFT)
2.2.3. Ring Opening Polymerization (ROP)
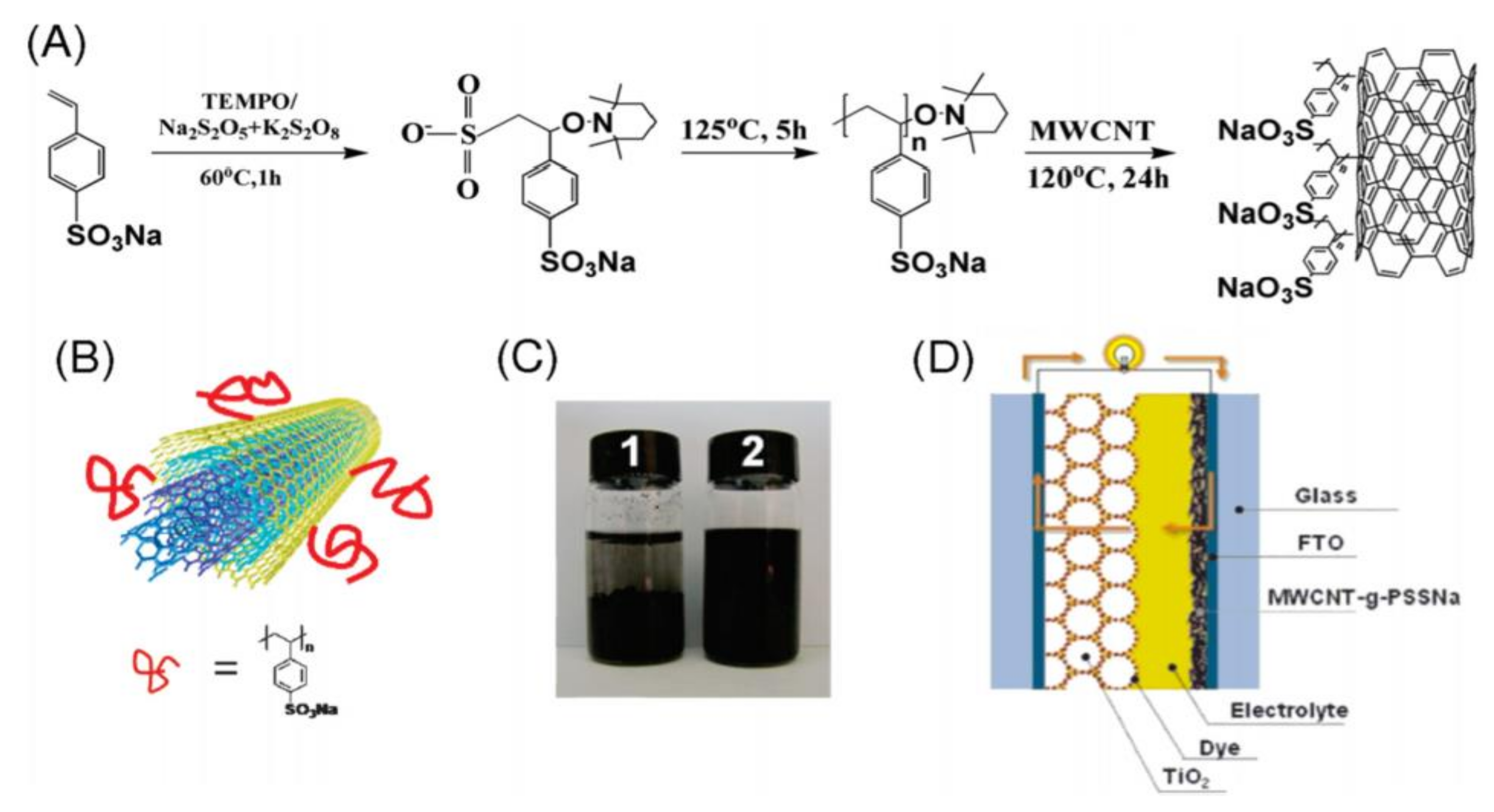
2.2.4. Free Radical Polymerization
3. Applications of Polymer-Functionalized CNTs
3.1. Biosensors and Biomedical Applications
3.2. Membranes
3.3. Energy Storage
3.4. EMI Shielding
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.Y. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Diez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Development of nanocomposites reinforced with carboxylated poly(ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 3729–3741. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, L.; Raimondo, M.; Naddeo, C.; Di Bartolomeo, A.; Lafdi, K. Influence of multiwall carbon nanotubes on morphological and structural changes during UV irradiation of syndiotactic polypropylene films. J. Polym. Sci. B Polym. Phys. 2012, 50, 963–975. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Di Bartolomeo, A.; Iemmo, L.; Zampino, D.C.; Vittoria, V.; Gorrasi, G. Ionic liquid as dispersing agent of LDH-carbon nanotubes into a biodegradable vinyl alcohol polymer. Polymers 2020, 12, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dervishi, E.; Li, Z.; Xu, Y.; Saini, V.; Biris, A.R.; Lupu, D.; Biris, A.S. Carbon nanotubes: Synthesis, properties, and applications. Part. Sci. Technol. 2009, 27, 107–125. [Google Scholar] [CrossRef]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon nanomaterials interfacing with neurons: An in vivo perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Pascual, A.M.; Naffakh, M.; Gómez, M.A.; Marco, C.; Ellis, G.; Martínez, M.T.; Ansón, A.; González-Domínguez, J.M.; Martínez-Rubi, Y.; Simard, B. Development and characterization of PEEK/carbon nanotube composites. Carbon 2009, 47, 3079–3090. [Google Scholar] [CrossRef] [Green Version]
- Tsang, S.C.; Chen, Y.K.; Harris, P.J.F.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, J.; Galiotis, C. Chemical oxidation of multi-walled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Anson-Casaos, A.; Díez-Pascual, A.M.; Ashrafi, B.; Naffakh, M.; Backman, D.; Stadler, H.; Johnston, A.; Gómez, M.; Matínez, M.T. Solvent-free preparation of high-toughness epoxy-SWNT composite materials. ACS Appl. Mater. Interfaces 2011, 3, 1441–1450. [Google Scholar] [CrossRef]
- Park, T.J.; Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Purification strategies and purity visualization techniques for single-walled carbon nanotubes. J. Mater. Chem. 2006, 16, 141–154. [Google Scholar] [CrossRef]
- Haddon, R.C.; Sippel, J.; Rinzler, A.G.; Papadimitrakopoulos, F. Purification and separation of carbon nanotubes. MRS Bull. 2004, 29, 241–252. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sarno, M.; Di Bartolomeo, A.; Sannino, D.; Ciambelli, P.; Vittoria, V. Incorporation of carbon nanotubes into polyethylene by high energy ball milling: Morphology and physical properties. J. Polym. Sci. B Polym. Phys. 2007, 45, 597–606. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, X.J.; Magniez, K.; Lamb, P.R.; de Celis Leal, D.R.; Fox, B.L.; Wang, X. Improving the mechanical properties of epoxy using multiwalled carbon nanotubes functionalized by a novel plasma treatment. Compos. Part A 2013, 45, 145–152. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Gómez, M.A.; Marco, C.; Ellis, G.; Martínez, M.T.; Ansón, A.; González-Domínguez, J.M.; Martínez-Rubi, Y.; Simard, B.; et al. The influence of a compatibilizer on the thermal and dynamic mechanical properties of PEEK/carbon nanotube composites. Nanotechnology 2009, 20, 315707. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lin, Y.; Taylor, S.; Gaillard, J.; Rao, A.M.; Sun, Y.P. Sonication-assisted functionalization and solubilization of carbon nanotubes. Nano Lett. 2002, 2, 231–234. [Google Scholar] [CrossRef]
- Williams, J.; Broughton, W.; Koukoulas, T.; Rahatekar, S. Plasma treatment as a method for functionalising and improving dispersion of carbon nanotubes in epoxy resins. J. Mater. Sci. 2013, 48, 1005–1013. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, S.B. Chemical Functionalization of Carbon Nanotubes. J. Nanosci. Nanotechnol. 2002, 2, 113–123. [Google Scholar] [CrossRef]
- Basheer, B.V.; George, J.J.; Siengchin, S.; Parameswaranpillai, J. Polymer grafted carbon nanotubes—Synthesis, properties, and applications: A review. Nano-Struct. Nano-Objects 2020, 22, 100429. [Google Scholar] [CrossRef]
- Sireesha, M.; Babu, V.J.; Kiran, A.S.K.; Ramakrishna, S.A. review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, J.M.; Castell, P.; Bespin-Gascon, S.; Anson-Casaos, A.; Diez-Pascual, A.M.; Gomez-Fatou, M.A.; Benito, A.M.; Maser, W.K.; Martinez, M.T. Covalent functionalization of MWCNTs with poly(p-phenylene sulphide) oligomers: A route to the efficient integration through a chemical approach. J. Mater. Chem. 2012, 22, 21285–21297. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Nish, A.; Hwang, J.Y.; Doig, J.; Nicholas, R.J. Highly selective dispersion of single-walled carbon nanotubes using aromatic polymers. Nat. Nanotechnol. 2007, 2, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xu, F.; Qiu, F.; Lu, H.; Yang, Y. Single-walled carbon nanotubes functionalized with high bonding density of polymer layers and enhanced mechanical properties of composites. Macromolecules 2007, 40, 3296–3305. [Google Scholar] [CrossRef]
- You, Y.Z.; Hong, C.Y.; Pan, C.Y. Functionalization of carbon nanotubes with well-defined functional polymers via thiol-coupling reaction. Macromol. Rapid Commun. 2006, 27, 2001–2006. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.M.; Zhao, H.; Yang, F.; Zhang, X. Surface modification of single-walled carbon nanotubes with polyethylene via in situ Ziegler–Natta polymerization. J. Appl. Polym. Sci. 2004, 92, 3697–3700. [Google Scholar]
- Zhang, Y.; Li, Q.; Wang, W.; Guo, A.; Li, J.; Li, H. Efficient and robust reactions for polyethylene covalently grafted carbon nanotubes. Macromol. Chem. Phys. 2017, 218, 1600449. [Google Scholar] [CrossRef]
- Baudot, C.; Volpe, M.V.; Kong, J.C.; Tan, C.M. Epoxy Functionalized Carbon Nanotubes and Methods of Forming the Same. U.S. Patent 299,082, 12 April 2009. [Google Scholar]
- Díez-Pascual, A.M.; Naffakh, M. Grafting of an aminated poly(phenylene sulphide) derivative to functionalized single-walled carbon nanotubes. Carbon 2012, 50, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhang, S.; Li, Y.; Li, J.; Liu, L.; Qin, Y.; Guo, Z.-X.; Dai, L.; Ye, C.; Zhu, D. PVK-modified single walled carbon nanotubes with effective photo induced electron transfer. Macromolecules 2003, 36, 6286–6288. [Google Scholar] [CrossRef]
- Blake, R.; Gun’ko, Y.K.; Coleman, J.; Cadek, M.; Fonseca, A.; Nagy, J.B.; Blau, W.J. A generic organometallic approach toward ultra-strong carbon nanotube polymer composites. J. Am. Chem. Soc. 2004, 126, 10226–10227. [Google Scholar] [CrossRef]
- Qin, S.; Qin, D.; Ford, W.T.; Resasco, D.E.; Herrera, J.E. Functionalization of single-walled carbon nanotubes with polystyrene via grafting to and grafting from methods. Macromolecules 2004, 37, 752–757. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Duft, A.M.; Adronov, A. Functionalization of single walled carbon nanotubes with well-defined polystyrene by “click” coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Haussler, M.; Lam, J.W.Y.; Dong, Y.; Wu, L. Synthesis of, light emission from, and optical power limiting in soluble single-walled carbon nanotubes functionalized by disubstituted polyacetylenes. J. Phys. Chem. B 2006, 110, 2302–2309. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, B.; Fernando, K.A.S.; Liu, P.; Allard, L.F.; Sun, Y.P. Polymeric carbon nanocomposites from carbon nanotubes functionalized with matrix polymer. Macromolecules 2003, 36, 7199–7204. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Martinez, G.; Gonzalez-Dominguez, J.M.; Martinez, T.; Gomez-Faou, M.A. Grafting of a hydroxylated PEEK derivative to the surface of single-walled carbon nanotubes. J. Mater. Chem. 2010, 20, 8285–8296. [Google Scholar] [CrossRef]
- Wang, T.Z.; Tseng, C.G. Polymeric carbon nanocomposites from multiwalled carbon nanotubes functionalized with segmented polyurethane. J. Appl. Polym. Sci. 2007, 105, 1642–1650. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.; Yu, F.; Fu, H.; Li, L.; Yan, G. Synthesis and characterization of ester bond-containing polyethyleneimine functionalized carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012002. [Google Scholar] [CrossRef]
- Kitano, H.; Tachimoto, K.; Anraku, Y.J. Functionalization of single-walled carbon nanotube by the covalent modification with polymer chains. J. Colloid Interfaces Sci. 2007, 306, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wie, J.; Kim, J. Thermal conductivity enhancement derived from poly(methyl methacrylate)-grafted carbon nanotubes in poly(methyl methacrylate)/polystyrene blends. Polymers 2019, 11, 1347. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Rao, A.M.; Sadanadan, B.; Kenik, E.A.; Sun, Y.P. Functionalizing multiple-walled carbon nanotubes with aminopolymers. J. Phys. Chem. B 2002, 106, 1294–1298. [Google Scholar] [CrossRef]
- Jung, Y.C.; Sahoo, N.G.; Cho, J.W. Polymeric nanocomposites of polyurethane block copolymers and functionalized multi-walled carbon nanotubes as crosslinkers. Macromol. Rapid. Commun. 2006, 27, 126–131. [Google Scholar] [CrossRef]
- Wu, H.L.; Yang, Y.T.; Ma, C.C.M.; Kuan, H.C. Molecular mobility of freeradical-functionalized carbon-nanotube/siloxane/poly(urea urethane) nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6084–6094. [Google Scholar] [CrossRef]
- Koshio, A.; Yudasaka, A.M.; Zhang, M.; Iijima, S. A simple way to chemically react single-wall carbon nanotubes with organic materials using ultrasonication. Nano Lett. 2001, 1, 361–363. [Google Scholar] [CrossRef]
- Kong, H.; Gao, C.; Yan, D. Controlled functionalization of multiwalled carbon nanotubes by in situ atom transfer radical polymerization. J. Am. Chem. Soc. 2004, 126, 412–413. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, C.; Jiang, H.; Zhang, L.; Zhu, X. Surface modification of carbon nanotubes by using iron-mediated activators generated by electron transfer for atom transfer radical polymerization. RSC Adv. 2018, 8, 11150–11156. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Braidy, N.; Botton, G.A.; Adronov, A. Polymerization from the surface of single-walled carbon nanotubes—preparation and characterization of nanocomposites. J. Am. Chem. Soc. 2003, 125, 16015–16024. [Google Scholar] [CrossRef]
- Baskaran, D.; Dunlap, J.R.; Mays, J.W.; Bratcher, M.S. Grafting efficiency of hydroxy-terminated poly(methyl methacrylate) with multiwalled carbon nanotubes. Macromol. Rapid. Commun. 2005, 26, 481–486. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Zhu, T.; Liu, Z. A general approach to chemical modification of single-walled carbon nanotubes with peroxy organic acids and its application in polymer grafting. J. Phys. Chem. C 2007, 111, 2379–2385. [Google Scholar] [CrossRef]
- Cui, J.; Wang, W.P.; You, Y.Z.; Liu, C.; Wang, P. Functionalization of multiwalled carbon nanotubes by reversible addition fragmentation chain-transfer polymerization. Polymer 2004, 45, 8717–8721. [Google Scholar] [CrossRef]
- Hong, C.Y.; You, Y.Z.; Pan, C.Y. Synthesis of water-soluble multiwalled carbon nanotubes with grafted temperature-responsive shells by surface RAFT polymerization. Chem. Mater. 2005, 17, 2247–2254. [Google Scholar] [CrossRef]
- Xu, G.; Wang, Y.; Pang, W.; Wu, W.-T.; Zhu, Q.; Wang, P. Fabrication of multiwalled carbon nanotubes with polymer shells through surface RAFT polymerization. Polym. Int. 2007, 56, 847–852. [Google Scholar] [CrossRef]
- Qu, L.; Veca, L.M.; Lin, Y.; Kitaygorodskiy, A.; Chen, B.; McCall, A.M.; Connell, J.W.; Sun, Y.-P. Soluble nylon-functionalized carbon nanotubes from anionic ring opening polymerization from nanotube surface. Macromolecules 2005, 38, 10328–10331. [Google Scholar] [CrossRef]
- Yang, M.; Gao, Y.; Li, H.; Adronov, A. Functionalization of multiwalled carbon nanotubes with polyamide 6 by anionic ring-opening polymerization. Carbon 2007, 45, 2327–2333. [Google Scholar] [CrossRef]
- Russell, K.E. Free radical graft polymerization and copolymerization at higher temperatures. Progress Polym. Sci. 2002, 27, 1007–1038. [Google Scholar] [CrossRef]
- Shaffer, M.S.P.; Koziol, K. Polystyrene grafted multi-walled carbon nanotubes. Chem. Commun. 2002, 2002, 2074–2075. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Wu, J.; Mai, Y.W. Synthesis and self-assembly of polystyrene-grafted multiwalled carbon nanotubes with a hairy-rod nanostructure. J. Polym. Sci. A Polym. Chem. 2006, 44, 3869–3881. [Google Scholar] [CrossRef]
- Qin, S.; Qin, D.; Ford, W.T.; Herrera, J.E.; Resasco, D.E.; Bachilo, S.M.; Weisman, R.B. Solubilization and purification of single-wall carbon nanotubes in water by in situ radical polymerization of sodium 4-styrenesulfonate. Macromolecules 2004, 37, 3965–3967. [Google Scholar] [CrossRef]
- Yue, B.; Wang, Y.; Huang, C.Y.; Pfeffer, R.; Iqbal, Z. Polymeric nanocomposites of functionalized carbon nanotubes synthesized in supercritical CO2. J. Nanosci. Nanotechnol. 2007, 7, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Soleyman, R.; Hirbod, S.; Adeli, M. Advances in the biomedical application of polymer-functionalized carbon nanotubes. Biomater. Sci. 2015, 3, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Q.; Tang, H.; Xie, Q.; Ma, M.; Tan, L.; Zhang, Y.; Yao, S. Characterization of and biomolecule immobilization on the biocompatible multi-walled carbon nanotubes generated by functionalization with polyamidoamine dendrimers. Colloids Surf. B 2010, 80, 18–25. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Chung, D.-J.; Kwen, H.-D. Fabrication of biosensors using vinyl polymer-grafted carbon nanotubes. In New Perspectives in Biosensors Technology and Applications; Intech Open: London, UK, 2011. [Google Scholar]
- Sianipar, M.; Kim, S.H.; Khoiruddin, K.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Xiao, P.; Chen, J.; Zhang, J.; Huang, Y.; Chen, T. Janus polymer/carbon nanotube hybrid membranes for oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 16204–16209. [Google Scholar] [CrossRef] [PubMed]
- Soyekwo, F.; Zhang, Q.; Chen, M.; Qu, Y.; Gao, R.; Lv, R.; Zhu, A.; Liu, Q.L. Metal in-situ surface functionalization of polymer-grafted-carbon nanotube composite membranes for fast efficient nanofiltration. J. Mater. Chem. A. 2017, 5, 583–592. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Lin, Y.F.; Weng, C.C.; Tsai, H.M.; Ma, C.C.M.; Lee, S.H.; Yen, M.Y.; Liu, P.I. Polypropylene-grafted multi-walled carbon nanotube reinforced polypropylene composite bipolar plates in polymer electrolyte membrane fuel cells. Energy Environ. Sci. 2011, 4, 543–550. [Google Scholar] [CrossRef]
- Han, H.; Kim, D.Y.; Jo, S.M.; Jang, S.Y. Water-soluble polyelectrolyte-grafted multiwalled carbon nanotube thin films for efficient counter electrode of dye-sensitized solar cells. ACS Nano 2010, 4, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Martínez, G.; Martinez, M.T.; Gómez, M.A. Novel nanocomposites reinforced with hydroxylated poly(ether ether ketone)-grafted carbon nanotubes. J. Mater. Chem. 2010, 20, 8247–8256. [Google Scholar] [CrossRef]
- Hayashida, K.; Matsuoka, Y. Electromagnetic interference shielding properties of polymer-grafted carbon nanotube composites with high electrical resistance. Carbon 2015, 85, 363–371. [Google Scholar] [CrossRef]
- Poothanari, M.A.; Abraham, J.; Kalarikkal, N.; Thomas, S. Excellent electromagnetic interference shielding and high electrical conductivity of compatibilized polycarbonate/ polypropylene carbon nanotube blend nanocomposites. Ind. Eng. Chem. Res. 2018, 57, 4287–4297. [Google Scholar] [CrossRef]
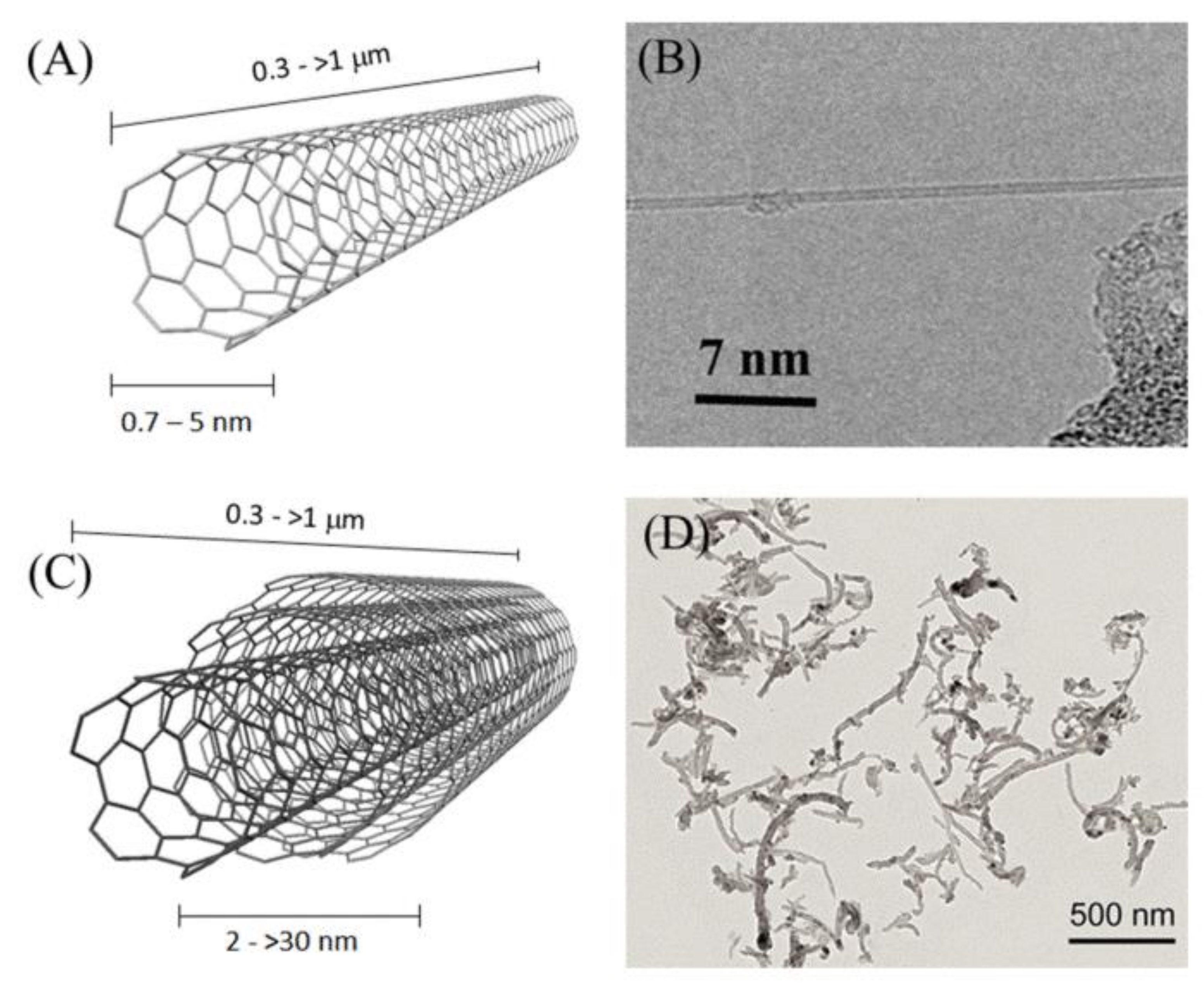



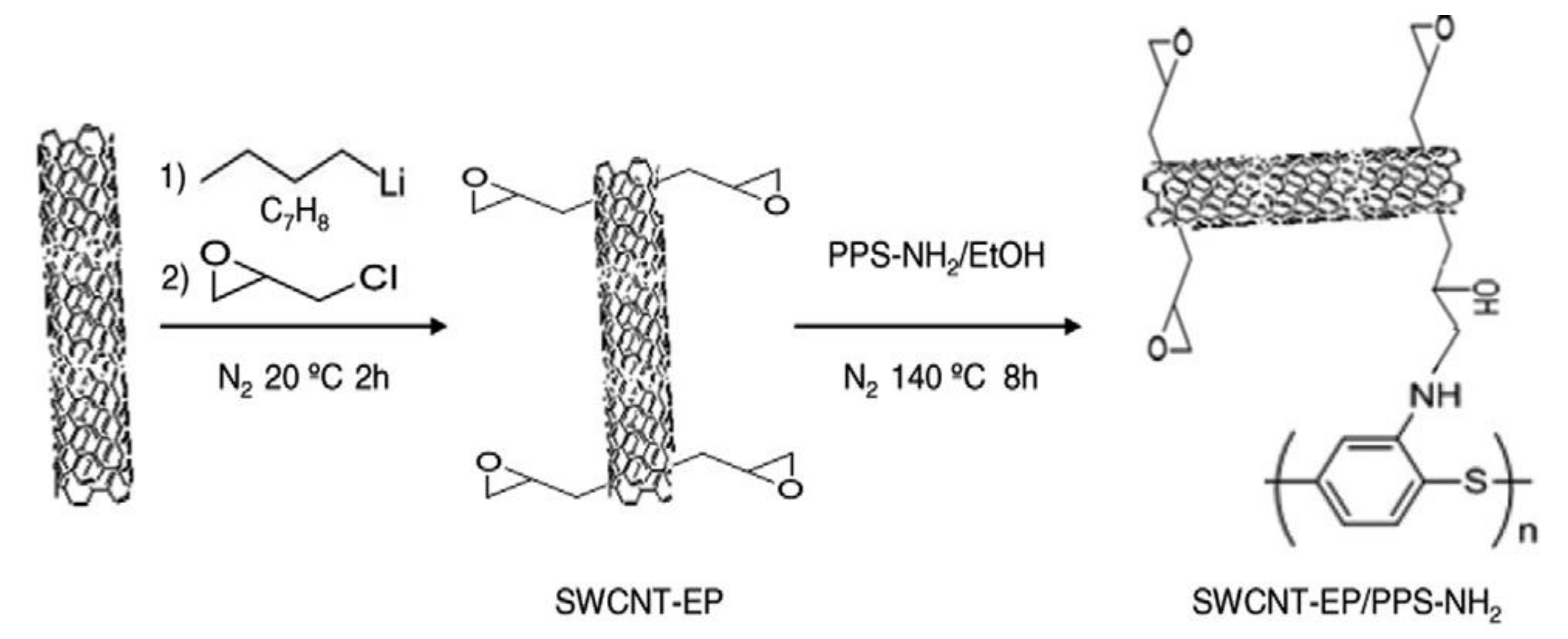

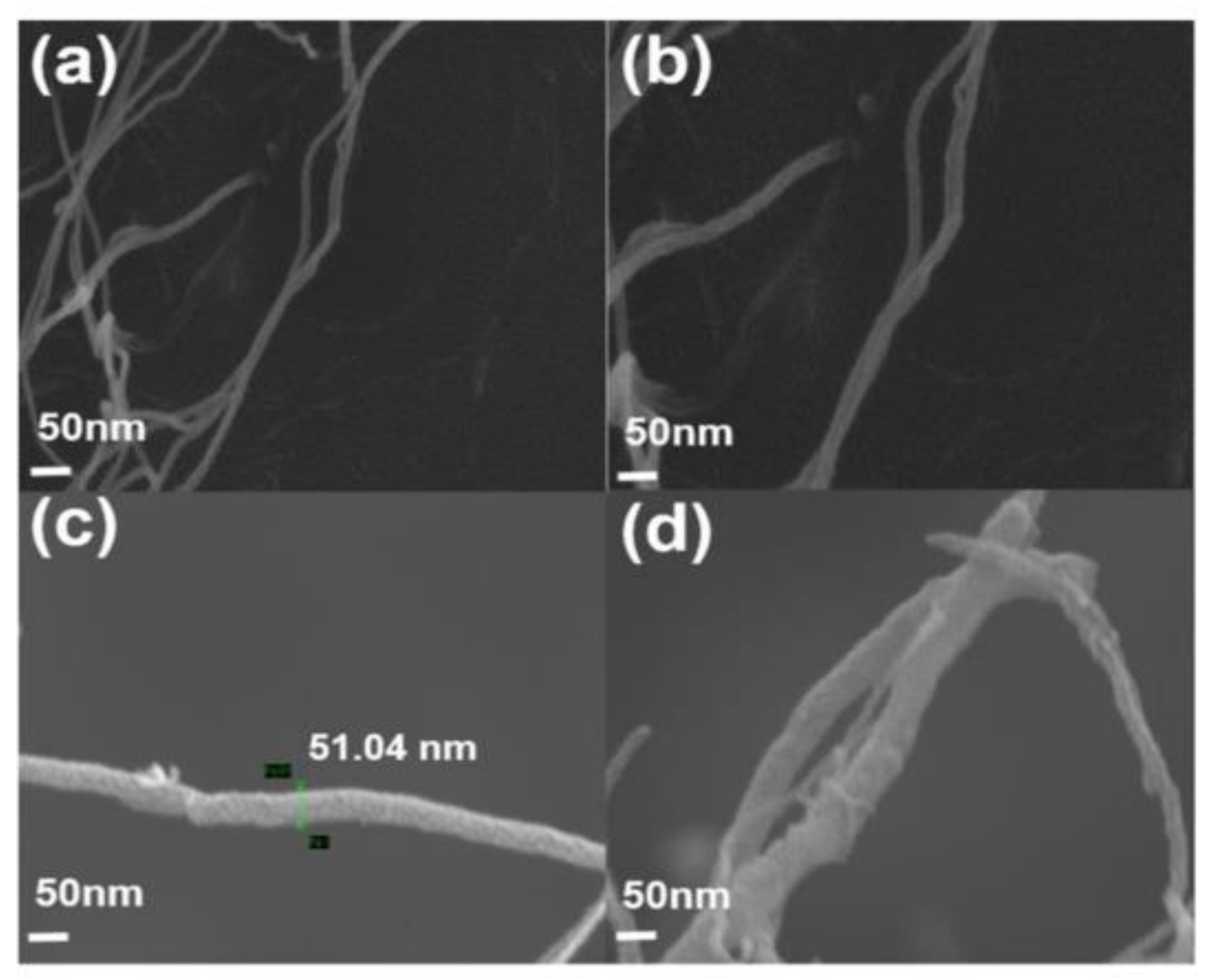
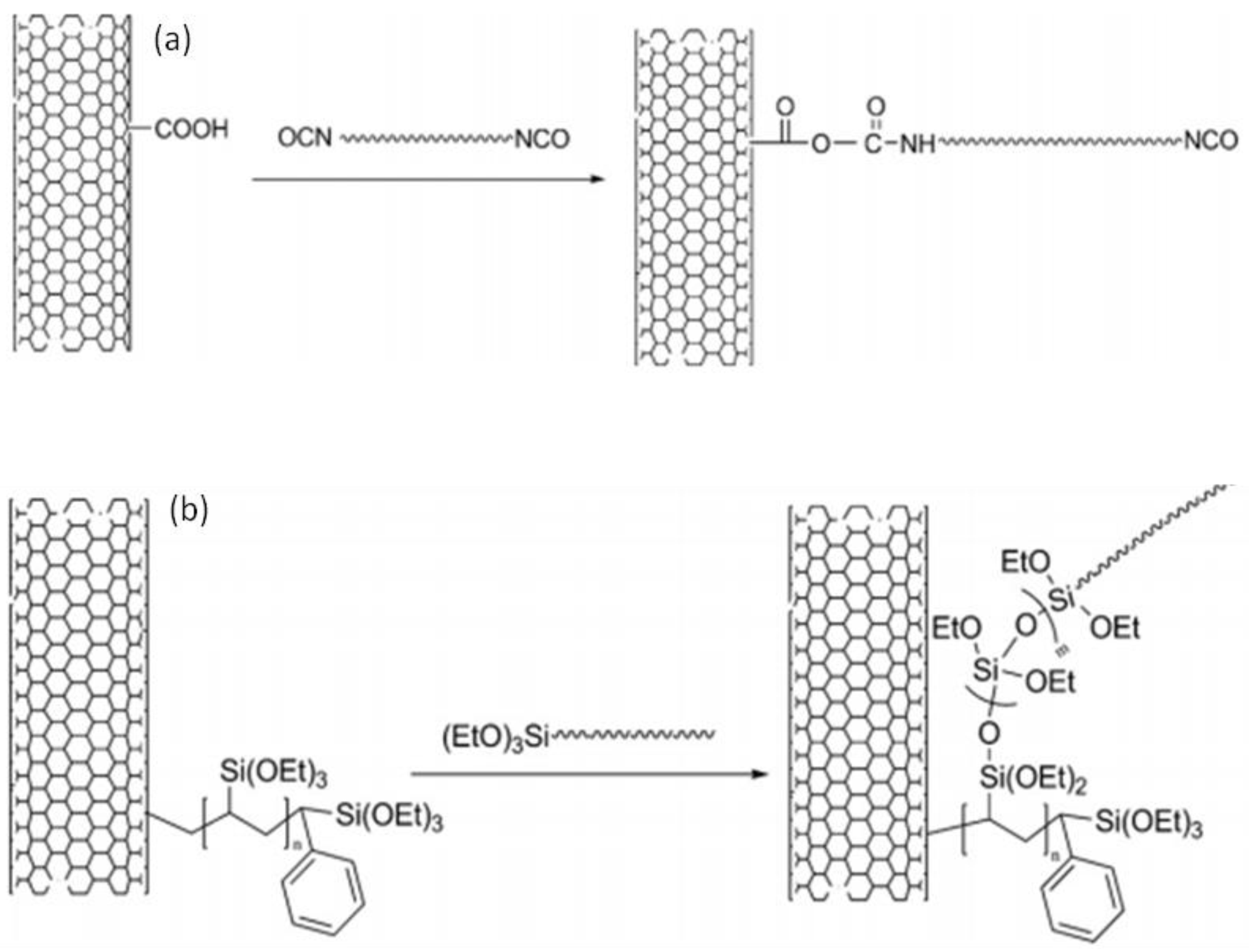
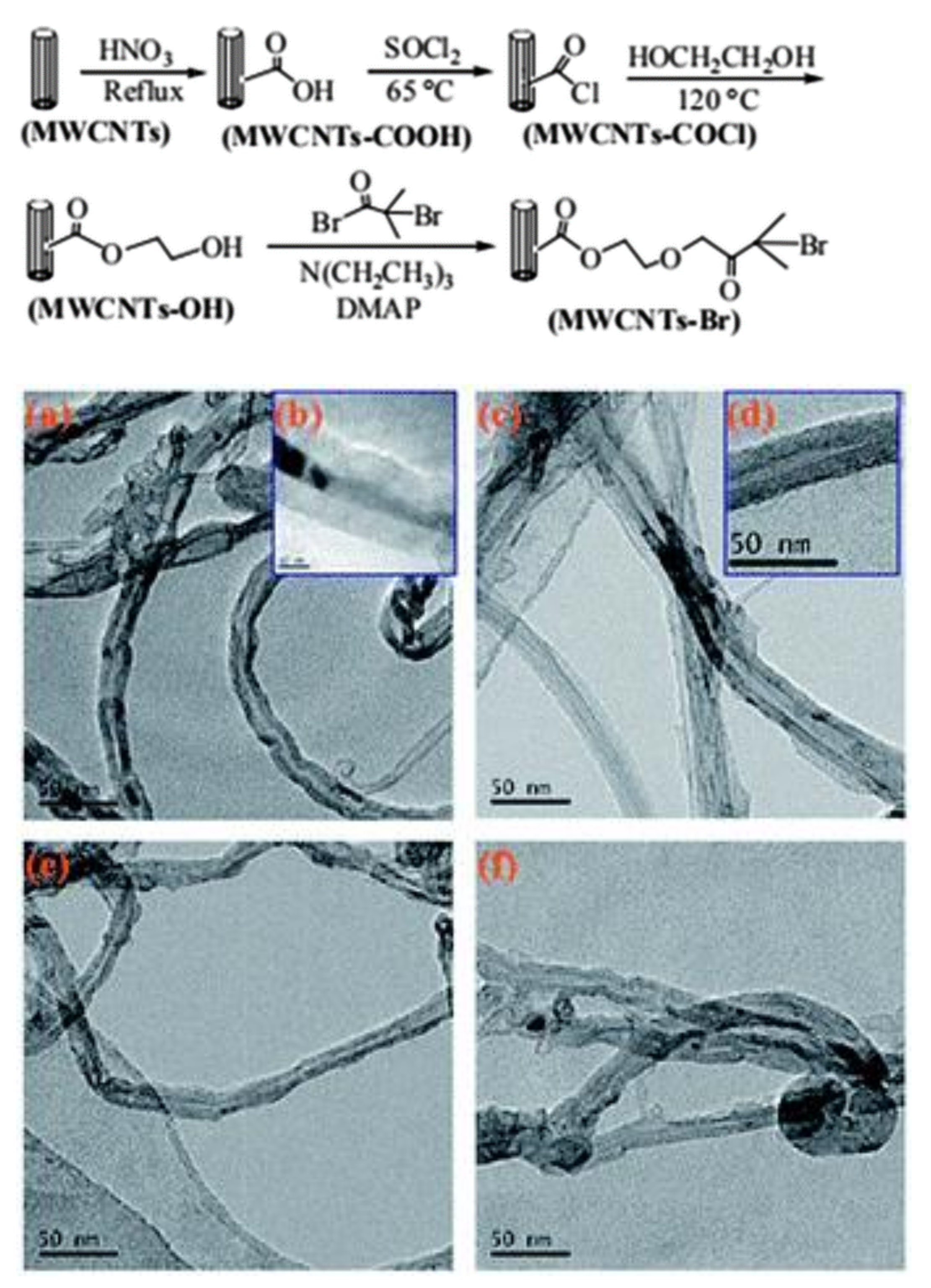
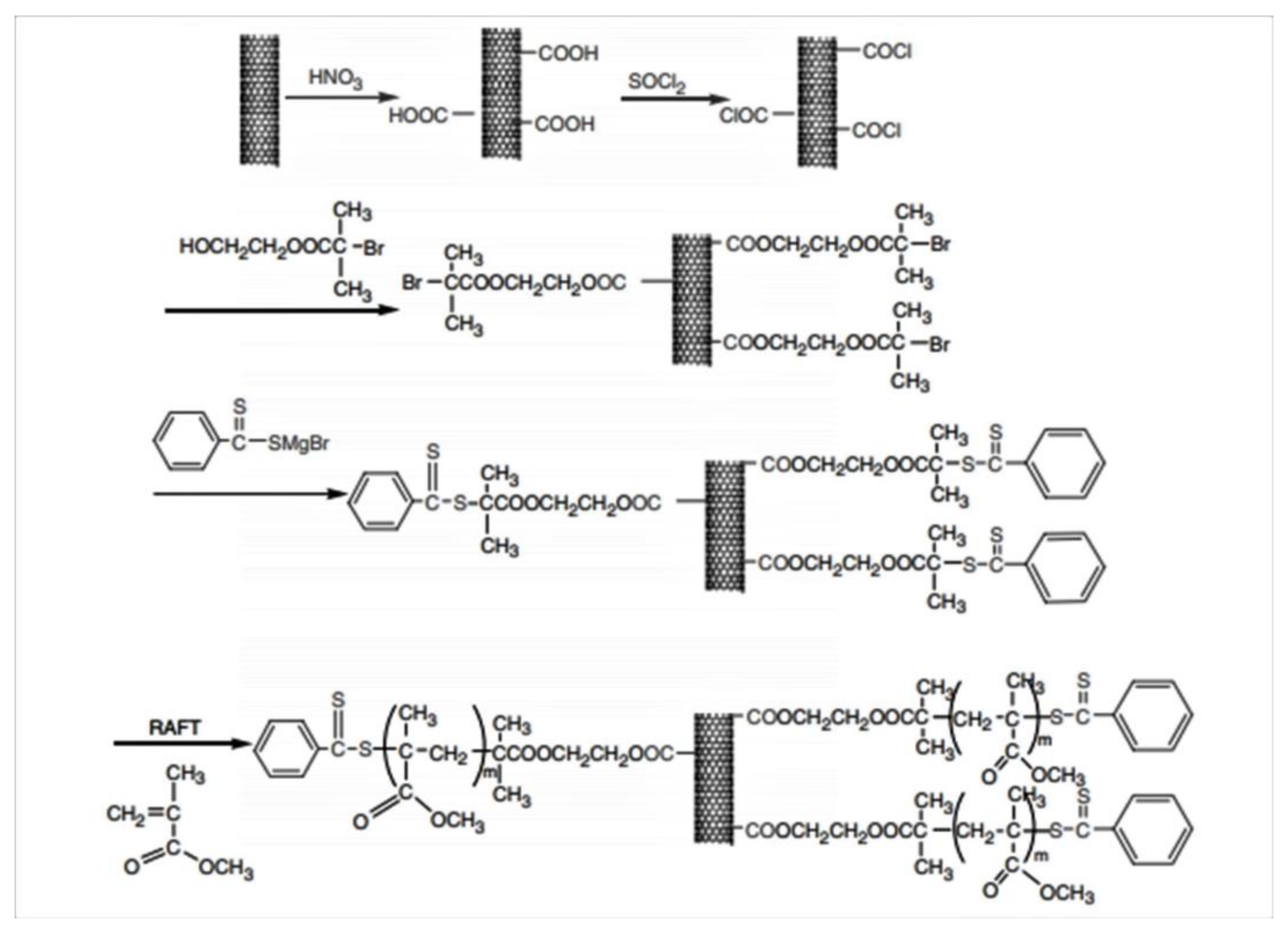
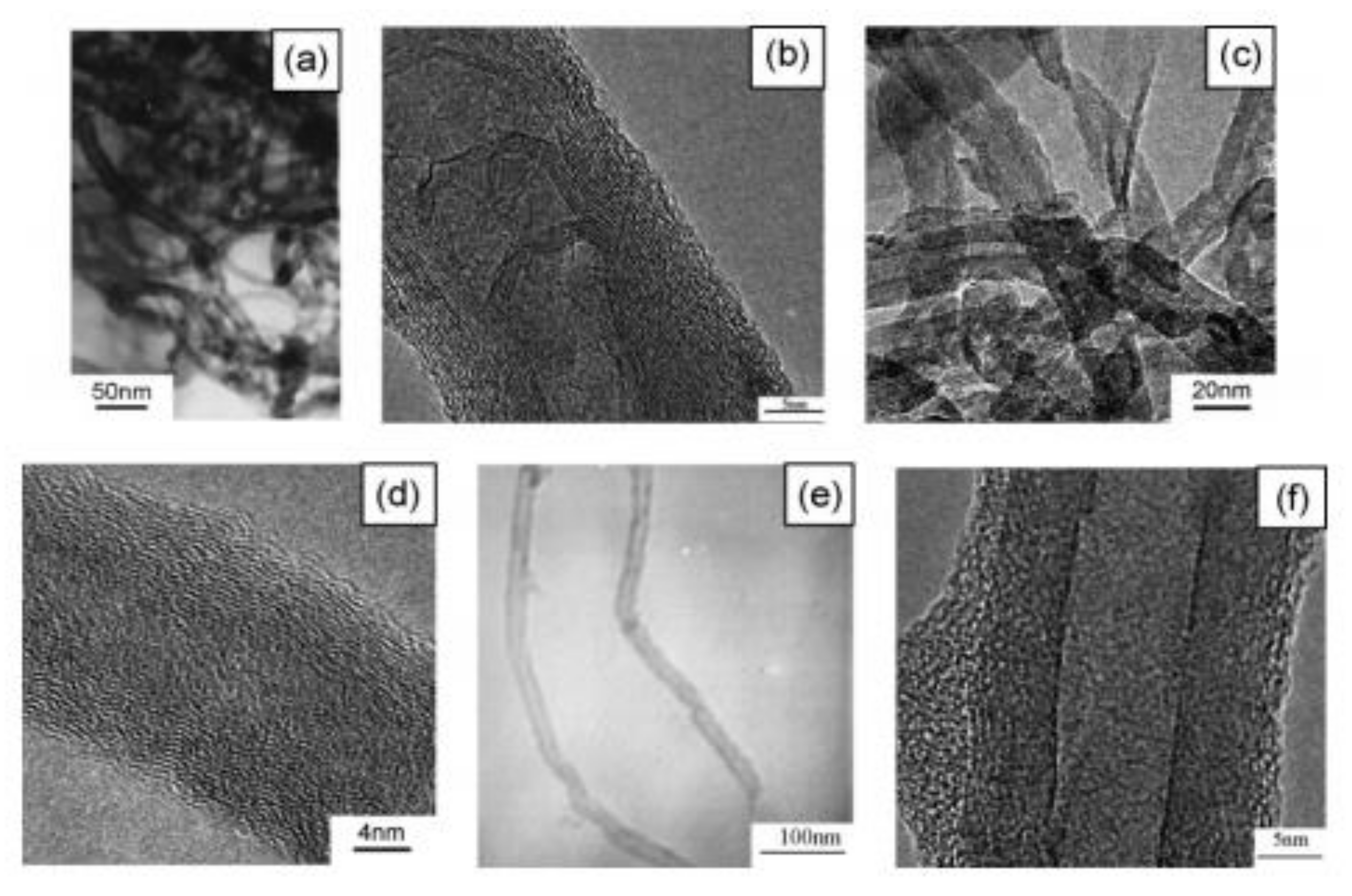

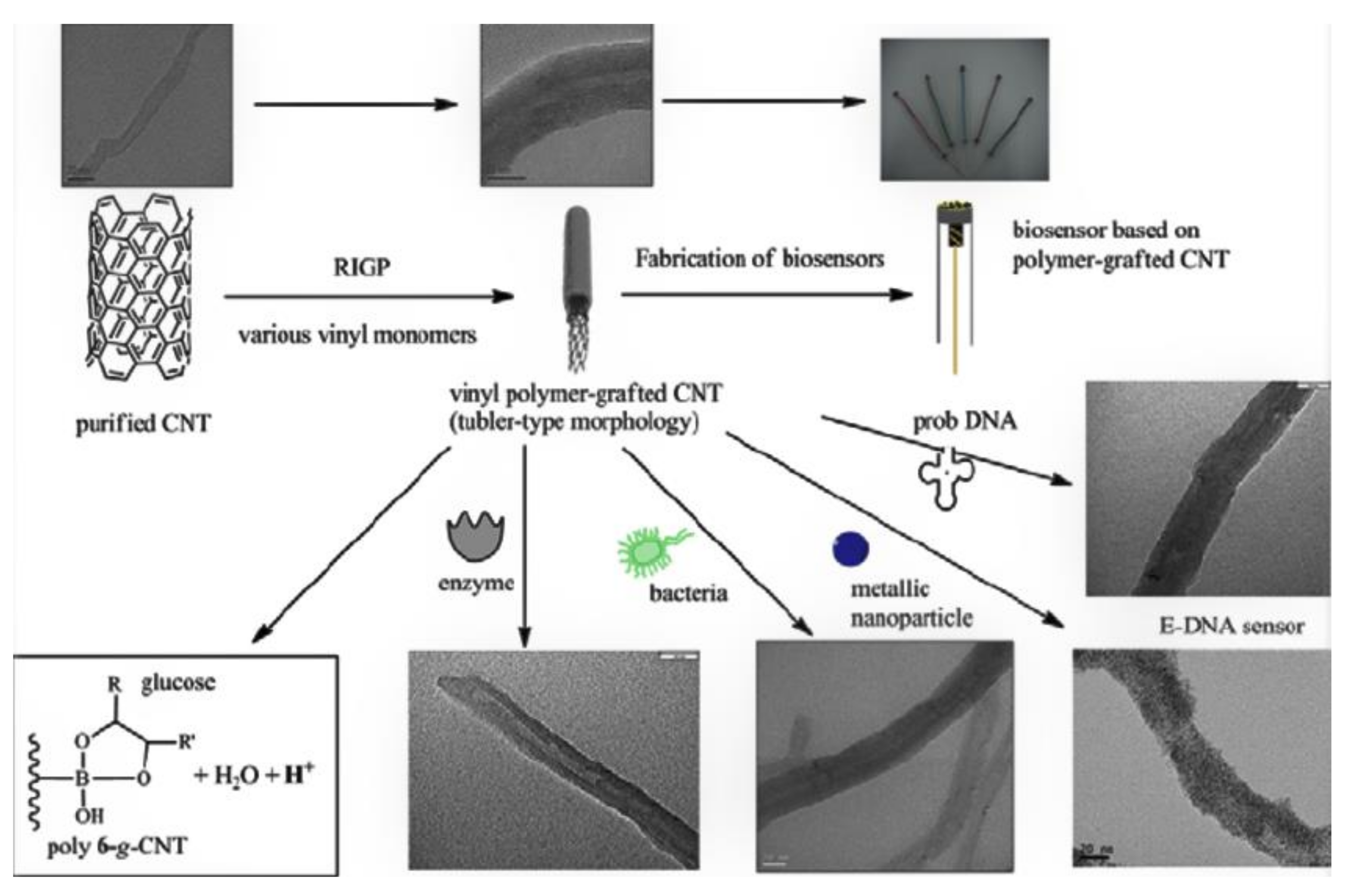
| Method | CNT Type | Polymer | Grafting Degree (%) | Ref. |
|---|---|---|---|---|
| Click coupling | alkyne-modified MWCNT | benzyl chlorinated polystyrene-co-poly(pchloromethylstyrene) | 53 | [28] |
| thiol-modified MWCNT | poly[N-(2-hydroxypropyl)methacrylamide] | [29] | ||
| oxidized MWCNT | polystyryllithium | 80 | [30] | |
| Nucleophilic addition | epoxy-modified SWCNT | aminated polyphenylene sulphide | 25 | [33] |
| acyl-choride-modified MWCNT | polystyryllithium | 40 | [34] | |
| Cycloaddition reaction | butyllithium-modified MWCNT | chlorinated polypropylene | 31 | [35] |
| SWCNT | azide-terminated PS | 2 | [36] | |
| alkyne-modified SWCNT | azide-terminated PS | 45 | [37] | |
| SWCNT | azide-terminated poly(diphenylacetylene) | 85 | [38] | |
| Amide and ester linkages | SWCNT/MWCNT | polyvinyl alcohol | [39] | |
| SWCNT | polyethylene glycol | 71 | [40] | |
| poly(aminobenzene sulfonic acid) | 30 | |||
| oxidized SWCNT | hydroxyl-modified polyetheretherketone | 12 | [41] | |
| carboxyl-modified MWCNT | polyethyleneimine | 25–36 | [43] | |
| MWCNT | poly(propionylethylenimine-co-ethylenimine) | 30–40 | [46] |
| Method | CNT Type | Polymer | Grafting Degree (%) | Ref. |
|---|---|---|---|---|
| ATRP | alkyl-modified MWCNT | poly(methyl methacrylate) | 32–82 | [50] |
| alkyl-modified SWCNT | poly(methyl methacrylate) | [52] | ||
| bromide-modified MWCNT | poly(methyl methacrylate) | 70 | [53] | |
| bromide-modified MWCNT | polystyrene | 18–33 | [54] | |
| SWCNT | poly(methyl methacrylate) | 17 | [54] | |
| RAFT | bromide-modified MWCNT | poly(methyl methacrylate) | 22–67 | [57] |
| ROP | isocyanate-modified MWCNTs | polyamide | 68 | [59] |
| Free radical polymerization | oxidized MWCNTs | polystyerene | 50–90 | [61] |
| SWCNTs | poly(sodium 4-styrenesulfonate) | 45 | [63] |
| Method | Advantages | Limitations |
|---|---|---|
| Click coupling | versatile, simple, high reaction rates low temperatures, easily removable byproducts | random coupling sites need metal catalyst some reagents are expensive |
| Nucleophilic addition | versatile, preformed commercial polymers can be used | prone to many side reactions nonuseful for composites insoluble in water |
| Cycloaddition reaction | versatile, one-pot synthesis | restricted to polymers with highly reactive groups need metal catalyst |
| Amide and ester linkages | Versatile, simple, mild conditions, short reaction time | expensive reagents require activation low grafting yield generate byproducts |
| ATRP | control of molecular weight, architecture, and composition low polydispersity | high catalyst concentration difficult to be performed in aqueous media |
| RAFT | versatile, mild conditions, useful for monomers soluble in water | need a specific RAFT agent need to optimize processing conditions |
| ROP | useful for high-molecular -weight polymers controlled chain length | slow, generate byproducts due to intermolecular reactions expensive |
| Free radical polymerization | versatile, simple, low cost | low selectivity, side reactions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64-83. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020006
Díez-Pascual AM. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol. 2021; 1(2):64-83. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020006
Chicago/Turabian StyleDíez-Pascual, Ana Maria. 2021. "Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview" Macromol 1, no. 2: 64-83. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020006






