Polymer-Derived Nitrogen-Doped Carbon Nanosheet Cluster and Its Application for Water Purification
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Polyazomethine
2.3. Material Characterization
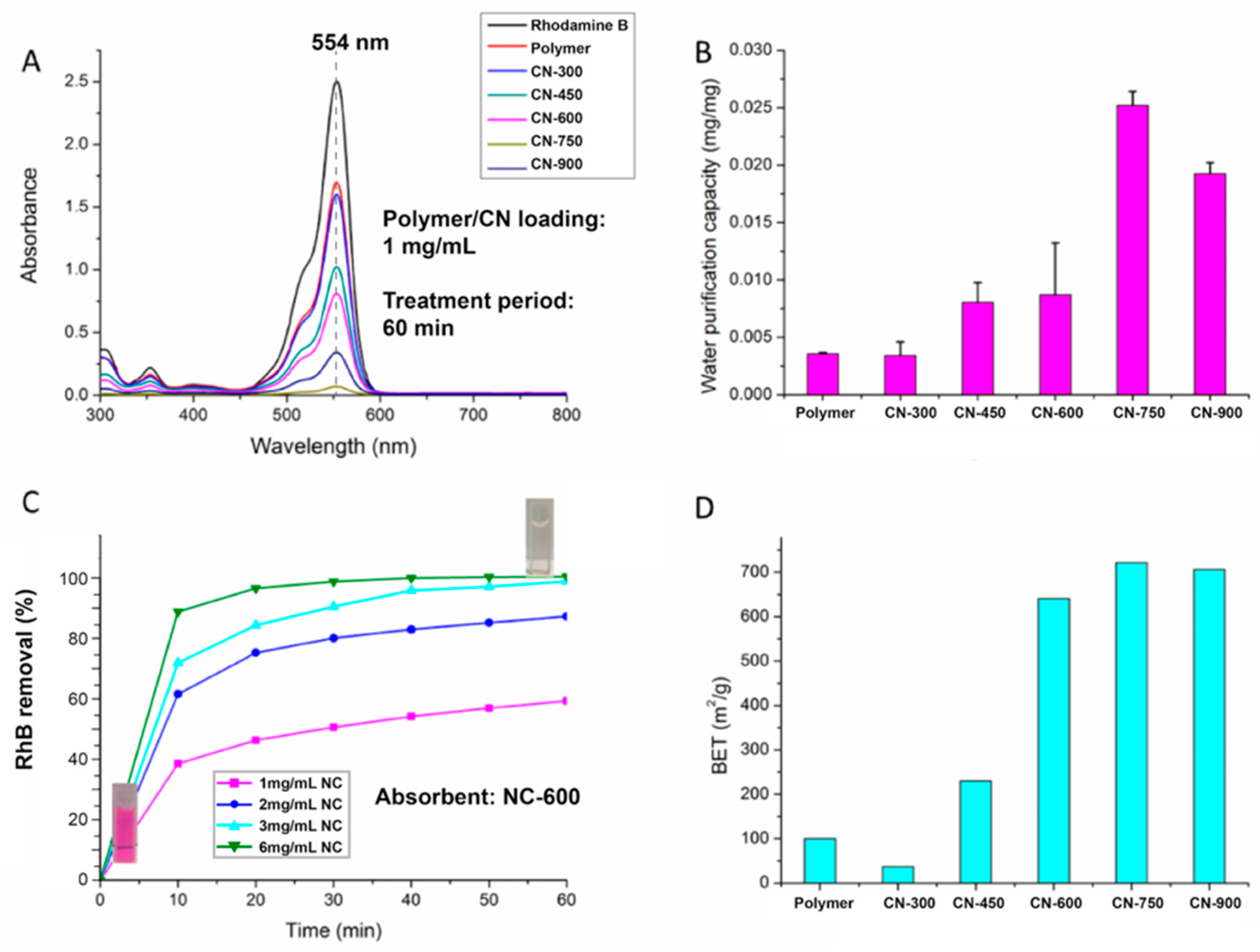
2.4. Water Purification Capacity Measurement
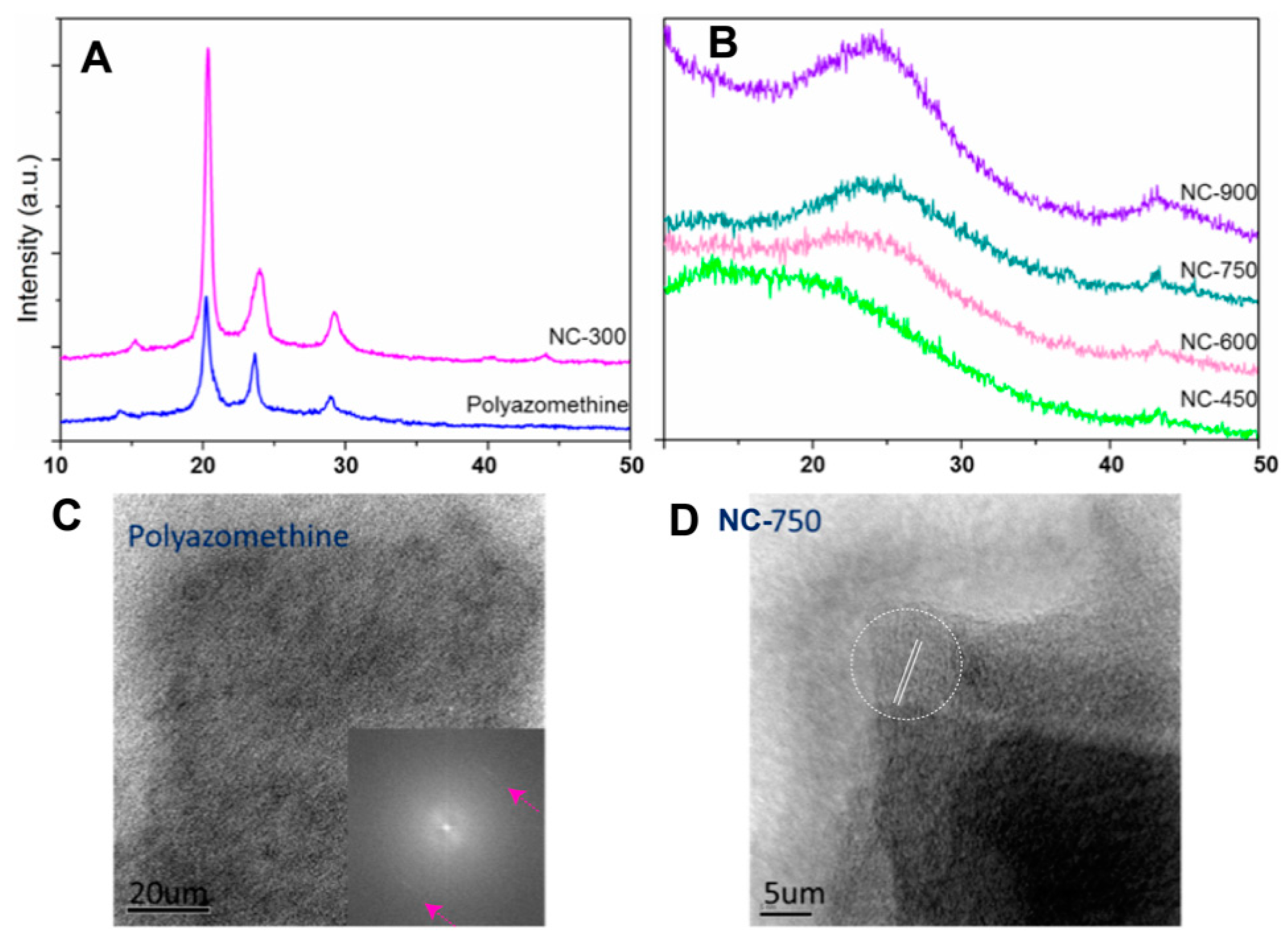
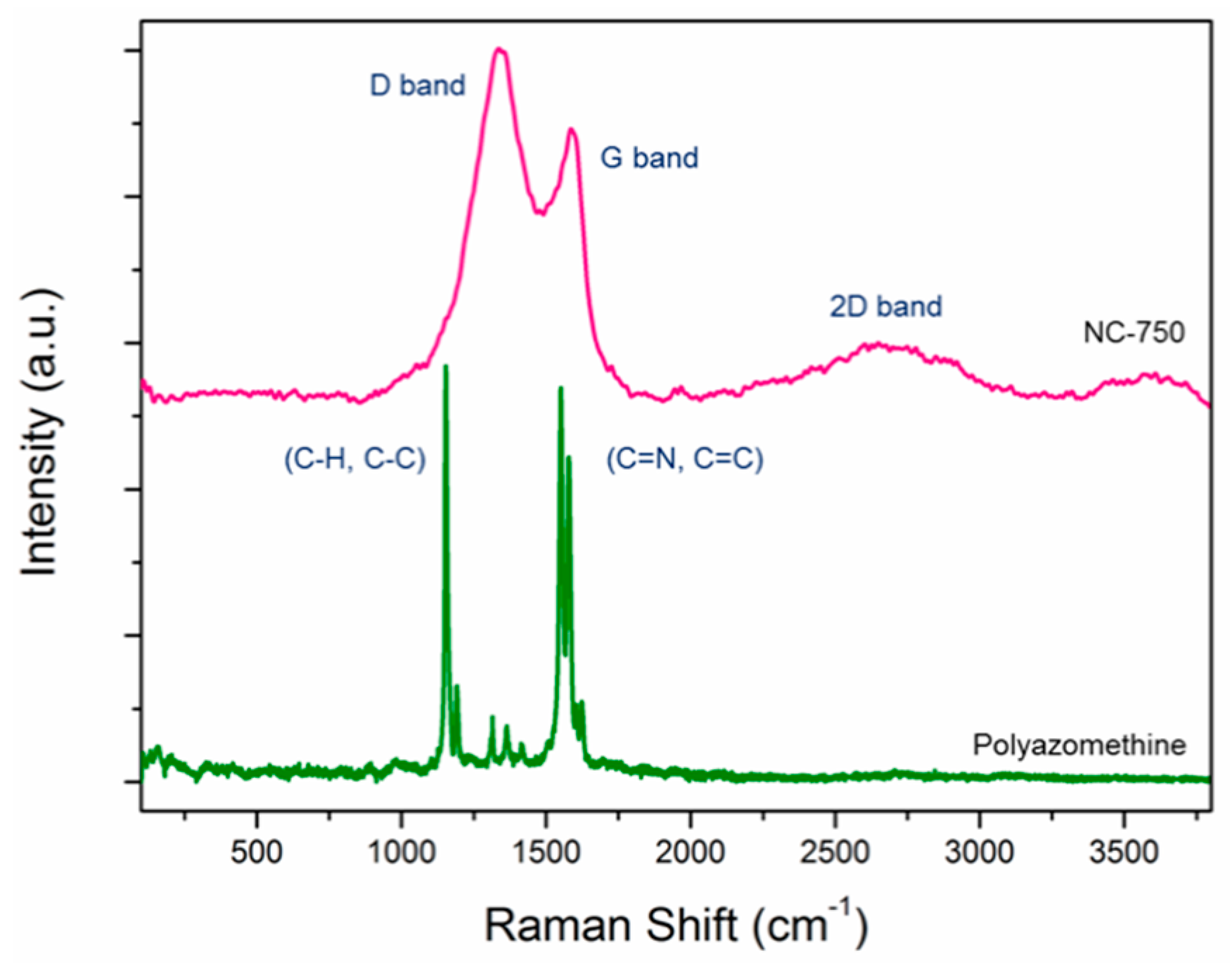
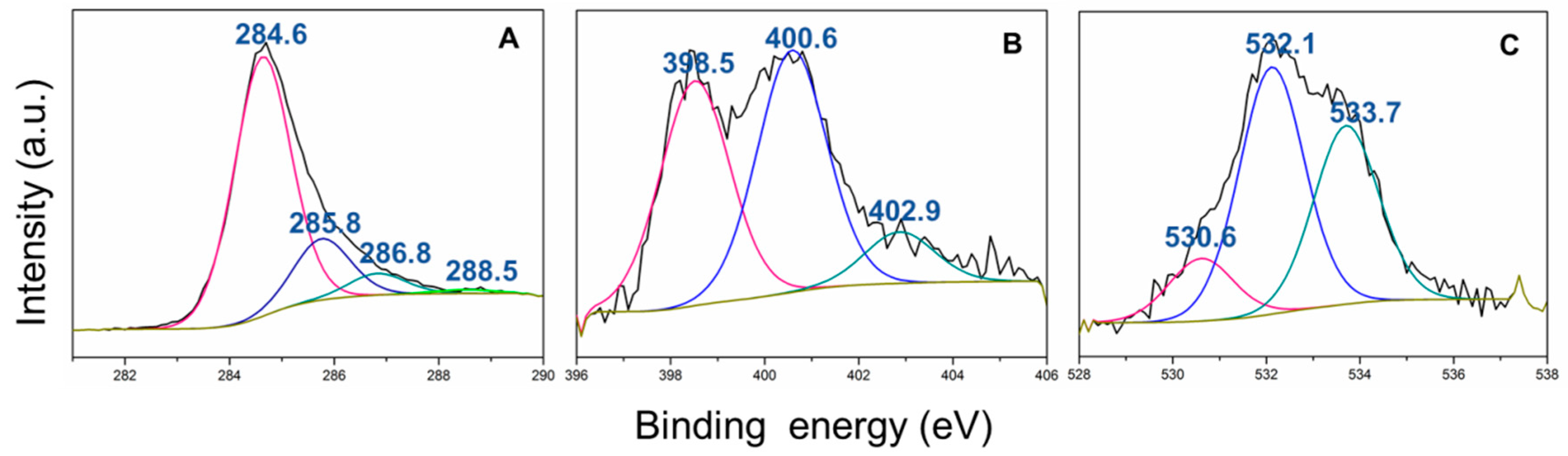
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhu, G.; Wang, M.; Li, J.; Lu, T.; Pan, L. Covalent-organic-frameworks derived N-doped porous carbon materials as anode for superior long-life cycling lithium and sodium ion batteries. Carbon 2017, 116, 686–694. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Fleming, E.; Du, F.; Ou, E.; Dai, L.; Shi, L. Thermal conductivity of carbon nanotubes grown by catalyst-free chemical vapor deposition in nanopores. Carbon 2019, 145, 195–200. [Google Scholar] [CrossRef]
- Sobrinho, R.A.L.; Andrade, G.R.S.; Costa, L.P.; de Souza, M.J.B.; de Souza, A.M.G.P.; Gimenez, I.F. Ordered micro-mesoporous carbon from palm oil cooking waste via nanocasting in HZSM-5/SBA-15 composite: Preparation and adsorption studies. J. Hazard. Mater. 2019, 362, 53–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, B.; Liu, L.; Zhao, Y.; Wu, H.; Qin, M.; Han, K.; Wang, W.A.; Xi, K.; Zhang, L. Hollow multihole carbon bowls: A stress–release structure design for high-stability and high-volumetric-capacity potassium-ion batteries. ACS Nano 2019, 13, 11363–11371. [Google Scholar] [CrossRef] [PubMed]
- Ariyanto, T.; Kurniasari, M.; Laksmana, W.; Prasetyo, I. Pore size control of polymer-derived carbon adsorbent and its application for dye removal. Int. J. Environ. Sci. Technol. 2019, 16, 4631–4636. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.-k.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-derived heteroatom-doped porous carbon materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mao, N.; Shuai, Q. Efficient removal of tetracycline from aqueous solution by covalent organic frameworks derived porous carbon. J. Environ. Chem. Eng. 2021, 9, 104842. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Hou, S.; Ma, J.; Lu, T.; Wang, J.; Yao, Y.; Pan, L. Facile dual doping strategy via carbonization of covalent organic frameworks to prepare hierarchically porous carbon spheres for membrane capacitive deionization. Chem. Commun. 2018, 54, 14009–14012. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Zhuang, J.-H.; Shirabe, K.; Yamashita, Y. Preparation of needle-like poly (azomethine) crystals by means of reaction-induced crystallization of oligomers. Polymer 2003, 44, 4761–4764. [Google Scholar] [CrossRef]
- Wang, J.; Senkovska, I.; Oschatz, M.; Lohe, M.R.; Borchardt, L.; Heerwig, A.; Liu, Q.; Kaskel, S. Imine-linked polymer-derived nitrogen-doped microporous carbons with excellent CO2 capture properties. ACS Appl. Mater. Interfaces 2013, 5, 3160–3167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q. An efficient one-step condensation and activation strategy to synthesize porous carbons with optimal micropore sizes for highly selective CO2 adsorption. Nanoscale 2014, 6, 4148–4156. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klock, C.; O’Keeffe, M.; Yaghi, O.M. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, L.; Dai, H.; Chen, Z.; Li, X.; Liu, X. Morphosynthesis of nanostructured polyazomethines and carbon through constitutional dynamic chemistry controlled reaction induced crystallization process. Polymer 2012, 53, 1611–1616. [Google Scholar] [CrossRef]
- Iwan, A.; Sek, D. Processible polyazomethines and polyketanils: From aerospace to light-emitting diodes and other advanced applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Łużny, W.; Stochmal-Pomarzańska, E.; Proń, A. Structural properties of selected poly (azomethines). Polymer 1999, 40, 6611–6614. [Google Scholar] [CrossRef]
- Gupta, S.S.; Sreeprasad, T.S.; Maliyekkal, S.M.; Das, S.K.; Pradeep, T. Graphene from sugar and its application in water purification. ACS Appl. Mater. Interfaces 2012, 4, 4156–4163. [Google Scholar] [CrossRef]
- Beams, R.; Cançado, L.G.; Novotny, L. Raman characterization of defects and dopants in graphene. J. Phys. Condens. Matter 2015, 27, 083002. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.C.; Kim, M.S.; Ryu, K.M.; Lee, S.H.; Jeong, Y.G. Microstructures and electrothermal characterization of aromatic poly (azomethine ether)-derived carbon films. J. Appl. Polym. Sci. 2020, 49345. [Google Scholar] [CrossRef]
- Bronnikov, S.; Kostromin, S.; Asandulesa, M.; Pankin, D.; Podshivalov, A. Interfacial interactions and interfacial polarization in polyazomethine/MWCNTs nanocomposites. Compos. Sci. Technol. 2020, 190, 108049. [Google Scholar] [CrossRef]
- Tseng, R.J.; Baker, C.O.; Shedd, B.; Huang, J.; Kaner, R.B.; Ouyang, J.; Yang, Y. Charge transfer effect in the polyaniline-gold nanoparticle memory system. Appl. Phys. Lett. 2007, 90, 053101. [Google Scholar] [CrossRef] [Green Version]
- Warta, C.L.; Papadimas, S.P.; Sorial, G.A.; Suidan, M.T.; Speth, T.F. The effect of molecular oxygen on the activated carbon adsorption of natural organic matter in Ohio river water. Water Res. 1995, 29, 551–562. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Wu, Y. Adsorption of toluene, ethylbenzene and m-xylene on multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. J. Hazard. Mater. 2011, 192, 1370–1379. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Wang, Y.; Gou, X.; Duan, Y. Adsorption and removal of rhodamine B from aqueous solution by tannic acid functionalized graphene. Colloids Surf. A Physicochem. Eng. Asp. 2015, 477, 35–41. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zheng, T.; Pilla, S. Polymer-Derived Nitrogen-Doped Carbon Nanosheet Cluster and Its Application for Water Purification. Macromol 2021, 1, 84-93. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020007
Yu X, Zheng T, Pilla S. Polymer-Derived Nitrogen-Doped Carbon Nanosheet Cluster and Its Application for Water Purification. Macromol. 2021; 1(2):84-93. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020007
Chicago/Turabian StyleYu, Xiaoyan, Ting Zheng, and Srikanth Pilla. 2021. "Polymer-Derived Nitrogen-Doped Carbon Nanosheet Cluster and Its Application for Water Purification" Macromol 1, no. 2: 84-93. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1020007





