Inter- and Intra-Hydrogen Bonding Strategy to Control the Fluorescence of Acylhydrazone-Based Conjugated Microporous Polymers and Their Application to Nitroaromatics Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
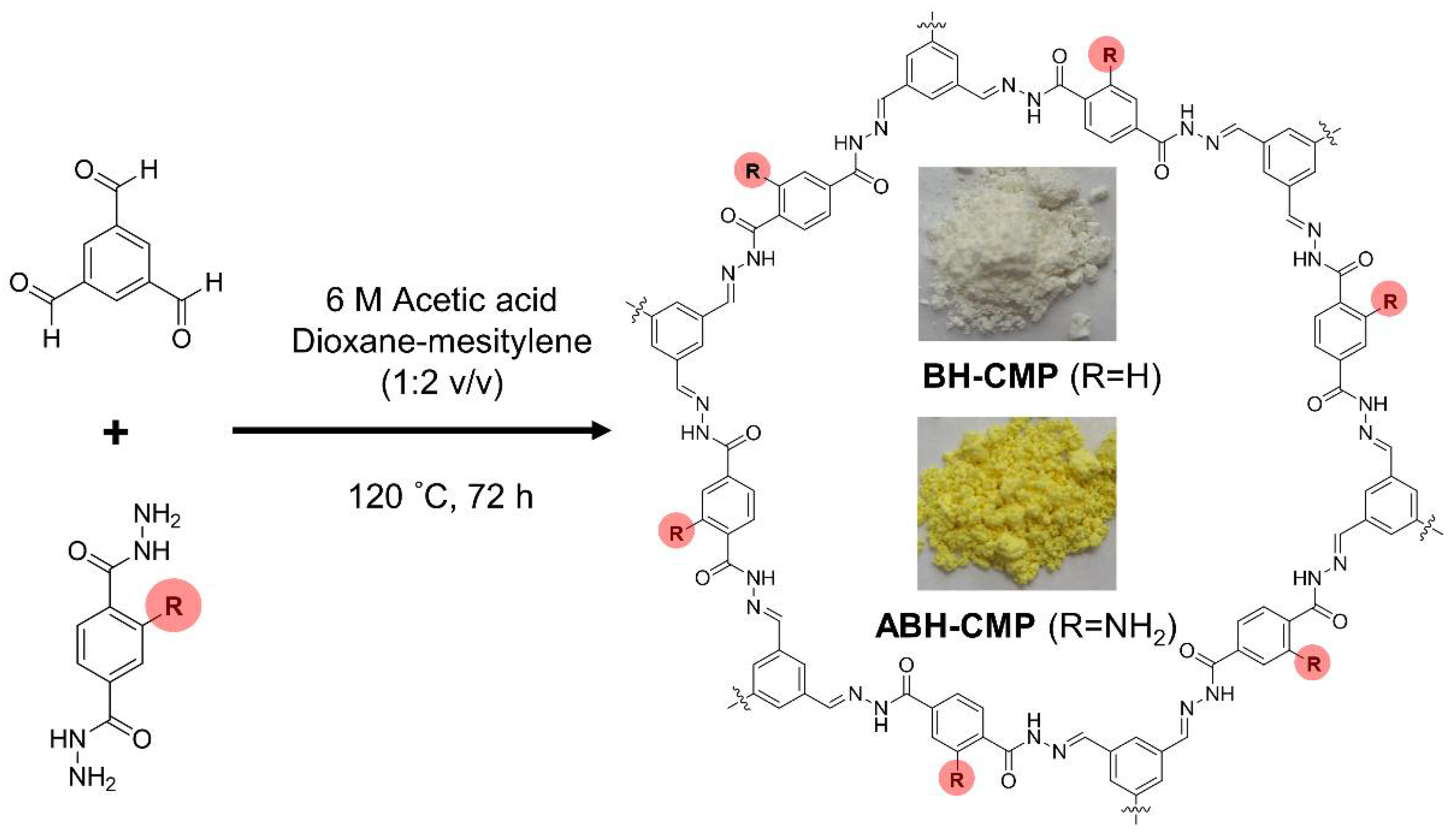
2.3. Synthesis
2.4. PL Measurements
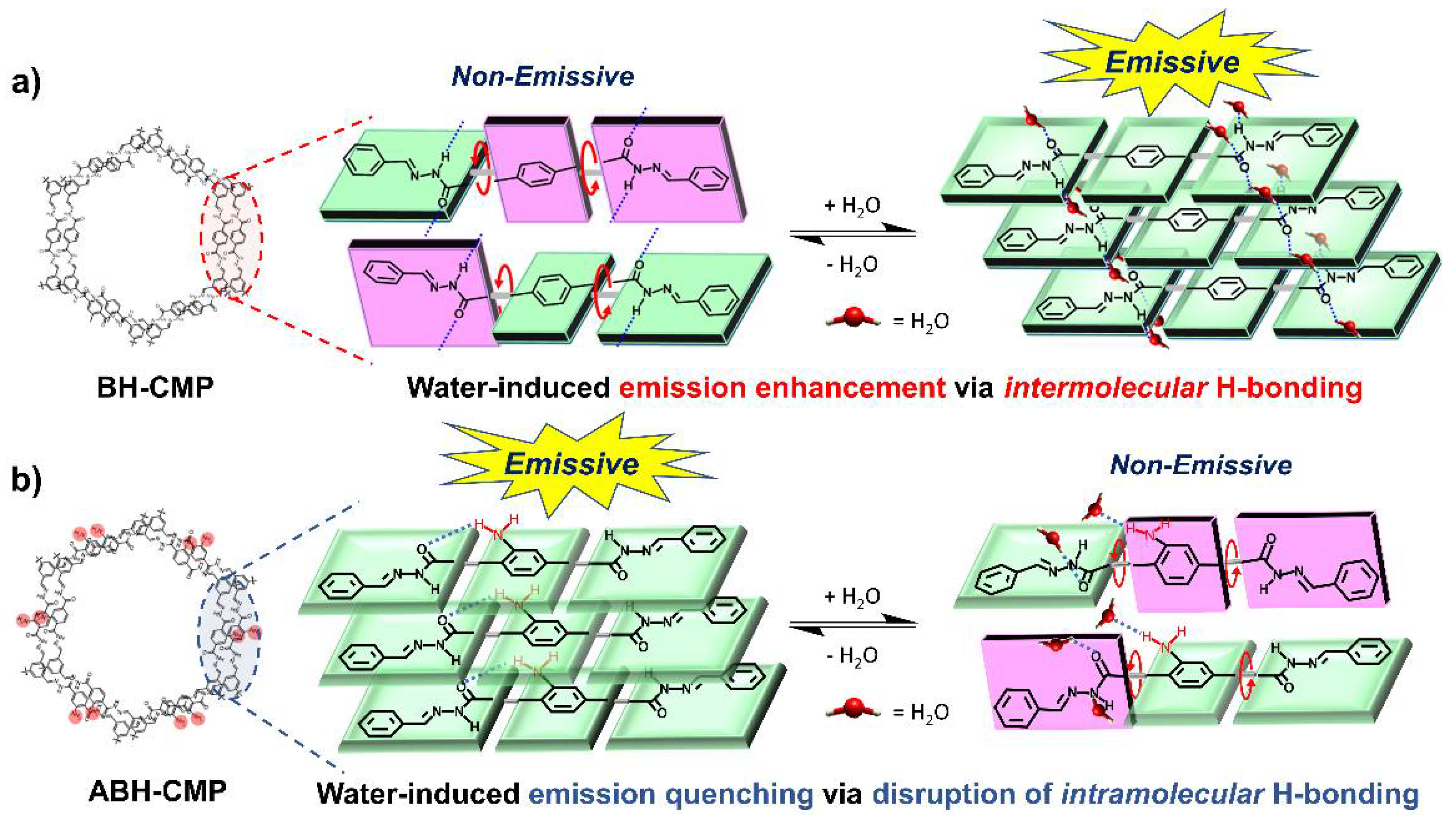
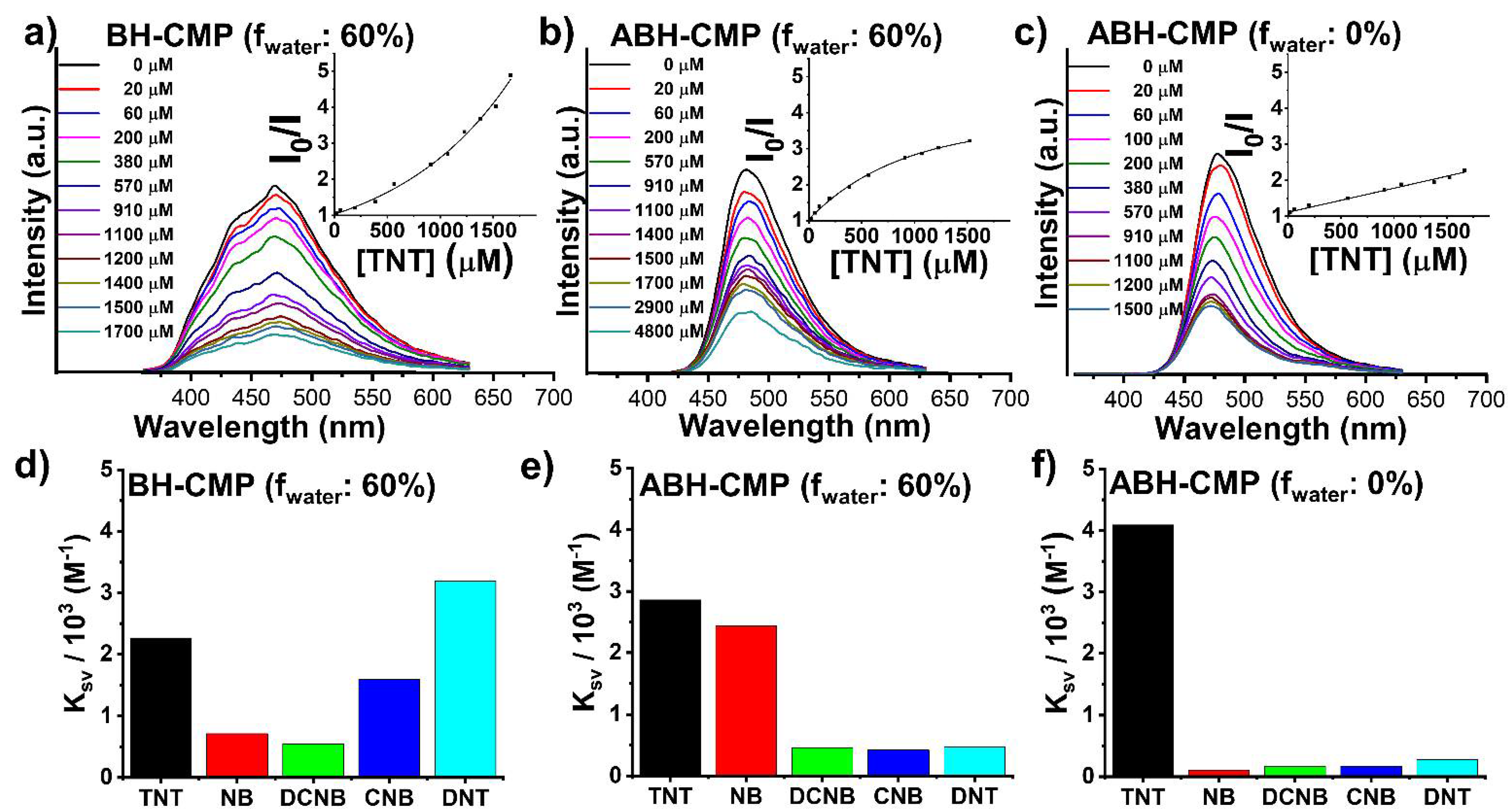
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.W.; Zhao, Z.; Cai, Y.; Xu, Z.; Yu, Y.; Xiong, Y.; Kwok, R.T.; Chen, Y.; Leung, N.L.; Ma, D. Facile access to deep red/near-infrared emissive AIEgens for efficient non-doped OLEDs. Chem. Sci. 2018, 9, 6118–6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Liu, B.; Zeng, J.; Nie, H.; Xiong, Y.; Zou, J.; Ning, H.; Wang, Z.; Zhao, Z.; Tang, B.Z. Efficient Bipolar Blue AIEgens for High-Performance Nondoped Blue OLEDs and Hybrid White OLEDs. Adv. Funct. Mater. 2018, 28, 1803369. [Google Scholar] [CrossRef]
- Tang, B.Z.; Qin, A. Aggregation-Induced Emission: Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-70177-5. [Google Scholar]
- Tang, Y.; Tang, B.Z. Principles and Applications of Aggregation-Induced Emission; Springer: New York, NY, USA, 2019; ISBN 978-3-319-99037-8. [Google Scholar]
- Wurthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Li, Y.H.; Xu, Z.; Zhu, X.Y.; Chen, B.; Wang, Z.M.; Xiao, B.; Lam, J.W.Y.; Zhao, Z.J.; Ma, D.G.; Tang, B.Z. Creation of Efficient Blue Aggregation-Induced Emission Luminogens for High-Performance Nondoped Blue OLEDs and Hybrid White OLEDs. ACS Appl. Mater. Inter. 2019, 11, 17592–17601. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Zhang, T.M.; Sun, J.; Wu, Y.L.; Liao, X.Q.; Lu, G.J.; Yang, J.J.; Wang, H.; Li, L.; Xu, B.S. Hyperbranched polymers with aggregation-induced emission property for solution-processed white organic light-emitting diodes. Tetrahedron 2018, 74, 7218–7227. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wang, B.; Li, D.; Yu, J. Fluorescent sensors based on AIEgen-functionalised mesoporous silica nanoparticles for the detection of explosives and antibiotics. Inorg. Chem. Front. 2018, 5, 2183–2188. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Cai, Y.; Kwok, R.T.; Hu, Y.; Lam, J.W.; Gu, X.; He, Z.; Zhao, Z.; Zheng, X. AIEgens for dark through-bond energy transfer: Design, synthesis, theoretical study and application in ratiometric Hg2+ sensing. Chem. Sci. 2017, 8, 2047–2055. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horiz. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Cao, D. Application of aggregation-induced emission (AIE) systems in sensing and bioimaging. Curr. Org. Chem. 2014, 18, 1028–1049. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Q.; Zhang, Z.; Qu, J.; Liu, R. Precise design and synthesis of an AIE fluorophore with near-infrared emission for cellular bioimaging. Mater. Sci. Eng. C 2018, 93, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; de Melo, J.S.S. Aggregation-Induced Emission: From Small Molecules to Polymers—Historical Background, Mechanisms and Photophysics. Top. Curr. Chem. 2021, 379, 379. [Google Scholar]
- Chiang, Y.-C.; Lai, Z.-L.; Chen, C.-M.; Chang, C.-C.; Liu, B. Construction of emission-tunable nanoparticles based on a TICT-AIEgen: Impact of aggregation-induced emission versus twisted intramolecular charge transfer. J. Mater. Chem. B 2018, 6, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Chen, L.-J.; Zhao, X.; Yan, X.-P. Enhancing near-infrared AIE of photosensitizer with twisted intramolecular charge transfer characteristics via rotor effect for AIE imaging-guided photodynamic ablation of cancer cells. Talanta 2021, 225, 122046. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.L.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.; Tang, B.Z. Restriction of intramolecular motions: The general mechanism behind aggregation-induced emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, E.; Lam, J.W.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, K.-S.; Kim, H.; Kim, T.; Malyutin, A.G.; Rees, D.C.; Yoo, B.-K.; Song, C. Microcrystal Electron Diffraction Elucidates Water-Specific Polymorphism-Induced Emission Enhancement of Bis-Arylacylhydrazone. ACS Appl. Mater. Int. 2021, 13, 7546–7555. [Google Scholar] [CrossRef]
- Yang, S.-J.; Ding, X.; Han, B.-H. Conjugated microporous polymers with extended π-structures for organic vapor adsorption. Macromolecules 2018, 51, 947–953. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in conjugated microporous polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Zhao, W.; Qiu, Q.; Xie, A.; Cheng, S.; Jiao, Y.; Pan, X.; Dong, W. Fluorescent conjugated microporous polymer (CMP) derived sensor array for multiple Organic/Inorganic contaminants detection. Sens. Actuators B Chem. 2020, 320, 128448. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Mo, W.; Tang, H.; Cheng, Z.; Chen, Y.; Zhang, S.; Ma, H.; Li, B.; Li, X. Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films for Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays. Adv. Funct. Mater. 2020, 30, 1910275. [Google Scholar] [CrossRef]
- Geng, T.-M.; Li, D.-K.; Zhu, Z.-M.; Guan, Y.-B.; Wang, Y. The synthesis and fluorescence detection properties of benzoquinone-based conjugated microporous/mesoporous polymers. Micropor. Mesopor. Mater. 2016, 231, 92–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Sigen, A.; Zou, Y.; Luo, X.; Li, Z.; Xia, H.; Liu, X.; Mu, Y. Gas uptake, molecular sensing and organocatalytic performances of a multifunctional carbazole-based conjugated microporous polymer. J. Mater. Chem. A 2014, 2, 13422–13430. [Google Scholar] [CrossRef]
- Masunaga, K.; Hayama, K.; Onodera, T.; Hayashi, K.; Miura, N.; Matsumoto, K.; Toko, K. Detection of aromatic nitro compounds with electrode polarization controlling sensor. Sens. Actuators B Chem. 2005, 108, 427–434. [Google Scholar] [CrossRef]
- Kulkarni, M.; Chaudhari, A. Microbial remediation of nitro-aromatic compounds: An overview. J. Environ. Manag. 2007, 85, 496–512. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.N.; Zhang, X.R.; Wang, S.F.; Fu, G.S.; Wang, J.L. Flatbands in 2D boroxine-linked covalent organic frameworks. Phys. Chem. Chem. Phys. 2016, 18, 1258–1264. [Google Scholar] [CrossRef]
- Spitler, E.L.; Koo, B.T.; Novotney, J.L.; Colson, J.W.; Uribe-Romo, F.J.; Gutierrez, G.D.; Clancy, P.; Dichtel, W.R. A 2D Covalent Organic Framework with 4.7-nm Pores and Insight into Its Interlayer Stacking. J. Am. Chem. Soc. 2011, 133, 19416–19421. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Stegbauer, L.; Hahn, M.W.; Jentys, A.; Savasci, G.; Ochsenfeld, C.; Lercher, J.A.; Lotsch, B.V. Tunable Water and CO2 Sorption Properties in Isostructural Azine-Based Covalent Organic Frameworks through Polarity Engineering. Chem. Mater. 2015, 27, 7874–7881. [Google Scholar] [CrossRef]
- Wu, S.F.; Gu, S.; Zhang, A.Q.; Yu, G.P.; Wang, Z.G.; Jian, J.G.; Pan, C.Y. A rational construction of microporous imide-bridged covalent-organic polytriazines for high-enthalpy small gas absorption. J. Mater. Chem. A 2015, 3, 878–885. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Liu, Y.Z.; Li, H.; Guan, X.Y.; Xue, M.; Yan, Y.S.; Valtchev, V.; Qiu, S.L.; Fang, Q.R. Three-Dimensional Mesoporous Covalent Organic Frameworks through Steric Hindrance Engineering. J. Am. Chem. Soc. 2020, 142, 3736–3741. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Q.; Wang, J.F.; Chen, Y.F.; Chen, Z.H.; Xu, H.S.; Tang, W.; Leng, K.; Ning, G.H.; Wu, J.S.; et al. Tuneable near white-emissive two-dimensional covalent organic frameworks. Nat. Commun. 2018, 9, 2335. [Google Scholar] [CrossRef] [PubMed]
- Miladinova, P.M.; Konstantinova, T.N. Synthesis and properties of some new blue-emitting triazine derivatives of 2-aminoterephthalic acid, containing a polymerisable group and stabiliser fragment. Color. Technol. 2009, 125, 242–247. [Google Scholar] [CrossRef]
- Kyprianou, D.; Berglund, M.; Emma, G.; Rarata, G.; Anderson, D.; Diaconu, G.; Exarchou, V. Synthesis of 2,4,6-Trinitrotoluene (TNT) Using Flow Chemistry. Molecules 2020, 25, 3586. [Google Scholar] [CrossRef]
- Zyryanov, G.V.E.; Kopchuk, D.S.; Kovalev, I.S.; Nosova, E.V.; Rusinov, V.L.; Chupakhin, O.N. Chemosensors for detection of nitroaromatic compounds (explosives). Russ. Chem. Rev. 2014, 83, 783. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Dong, W.Y.; Pina, J.; Pan, Y.Y.; Preis, E.; de Melo, J.S.S.; Scherf, U. Polycarbazoles and polytriphenylamines showing aggregation-induced emission (AIE) and intramolecular charge transfer (ICT) behavior for the optical detection of nitroaromatic compounds. Polymer 2015, 76, 173–181. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, I.; Baek, S.; Song, S.G.; Kim, J.; Lee, H.K.; Lee, J.; Kim, K.-s.; Song, C. Inter- and Intra-Hydrogen Bonding Strategy to Control the Fluorescence of Acylhydrazone-Based Conjugated Microporous Polymers and Their Application to Nitroaromatics Detection. Macromol 2021, 1, 234-242. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030016
Cha I, Baek S, Song SG, Kim J, Lee HK, Lee J, Kim K-s, Song C. Inter- and Intra-Hydrogen Bonding Strategy to Control the Fluorescence of Acylhydrazone-Based Conjugated Microporous Polymers and Their Application to Nitroaromatics Detection. Macromol. 2021; 1(3):234-242. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030016
Chicago/Turabian StyleCha, Inhwan, Seohyun Baek, Sun Gu Song, Junggong Kim, Ho Keun Lee, Jongman Lee, Kyung-su Kim, and Changsik Song. 2021. "Inter- and Intra-Hydrogen Bonding Strategy to Control the Fluorescence of Acylhydrazone-Based Conjugated Microporous Polymers and Their Application to Nitroaromatics Detection" Macromol 1, no. 3: 234-242. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030016





