Aspects of the Synthesis of Poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated Cationic Polymerization in Open Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization Procedures
2.3. Methods
3. Results
3.1. Polymerization of Isobutylene
3.1.1. Effect of Proton Trap Nature and Initiator Concentration
3.1.2. Effect of Proton Trap Concentration
3.2. Synthesis of Triblock Copolymers
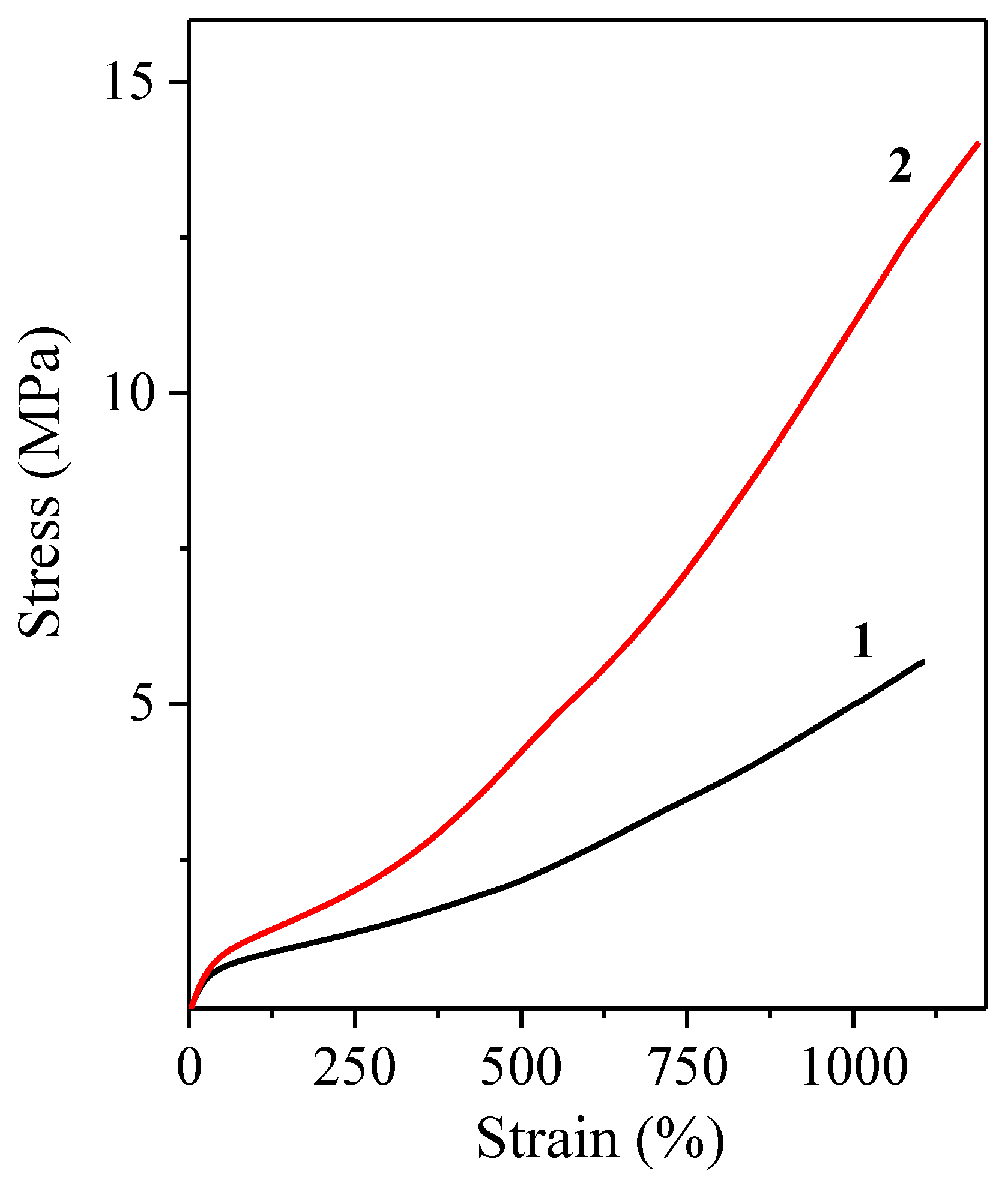
3.3. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puskas, J.E.; Kaszas, G. Polyisobutylene-based thermoplastic elastomers: A review. Rubber Chem. Technol. 1966, 69, 462–475. [Google Scholar] [CrossRef]
- Puskas, J.E.; Antony, P.; Kwon, Y.; Paulo, C.; Kovar, M.; Norton, P.R.; Kaszas, G.; Altstadt, V. Macromolecular engineering via carbocationic polymerization: Branched structures, block copolymers and nanostructures. Macromol. Mater. Eng. 2001, 286, 565–582. [Google Scholar] [CrossRef]
- Kennedy, J.P. From thermoplastic elastomers to designed biomaterials. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2951–2963. [Google Scholar] [CrossRef]
- Puskas, J.E.; Antony, P.; Fray, M.; Altstadt, V. The effect of hard and soft segment composition and molecular architecture on the morphology and mechanical properties of polystyrene-polyisobutylene thermoplastic elastomeric block copolymers. Eur. Polym. J. 2003, 39, 2041–2049. [Google Scholar] [CrossRef]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephjerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Puskas, J.E.; Chen, Y.; Dahman, Y.; Padavan, D. Polyisobutylene-based biomaterials. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3091–3109. [Google Scholar] [CrossRef]
- Basak, S. Thermoplastic elastomers in biomedical industry—evolution and current trends. J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Akentieva, T.; Shishkova, D.; Krivikina, E.; Klyshnikov, K.; et al. Polyisobutylene-based thermoplastic elastomers for manufacturing polymeric heart valve leaflets: In vitro and in vivo results. Appl. Sci. 2019, 9, 4773. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.B.; Li, K.; Xiang, D.; Zhang, M.; Yang, D.; Zhang, J.H.; Mao, J.; Wang, H.; Guo, W.L. Surface immobilization of heparin on functional polyisobutylene-based thermoplastic elastomer as a potential artificial vascular graft. Appl. Surf. Sci. 2018, 445, 8–15. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Yuzhalin, A.E.; Glushkova, T.V.; Makarevich, M.I.; Nikishau, P.A.; Kostjuk, S.V.; Klyshnikov, K.Y.; Matveeva, V.G.; Khanova, M.Y.; Ovcharenko, E.A. Biocompatible nanocomposites based on poly(styrene-block-isobutylene-block-styrene) and carbon nanotubes for biomedical application. Polymers 2020, 12, 2158. [Google Scholar] [CrossRef]
- Mo, Z.H.; Wu, Y.-X. Arc-bridge Polydimethylsiloxane grafted graphene incorporation into quaternized poly(styrene-b-isobutylene-b-styrene) for construction of anion exchange membranes. Polymer 2019, 177, 290–297. [Google Scholar] [CrossRef]
- Yang, R.; PDai, P.; Zhang, S.; Xu, R.-W.; Hong, S.; Lin, W.-F.; Wu, Y.-X. In-situ synthesis of cross-linked imidazolium functionalized poly(styrene-b-isobutylene-b-styrene) for anion exchange membranes. Polymer 2021, 224, 123682. [Google Scholar] [CrossRef]
- Kwon, Y.; Faust, R. Synthesis of polyisobutylene-based block copolymers with precisely controlled architecture by living cationic polymerization. Adv. Polym. Sci. 2004, 167, 107–135. [Google Scholar]
- Dey, A.; Haldar, U.; De, P. Block copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front. Chem. 2021, 9, 644547. [Google Scholar] [CrossRef]
- Kaszas, G.; Puskas, J.E.; Kennedy, J.P.; Hager, W.G. Polyisobutylene-containing block polymers by sequential monomer addition. II. Polystyrene–polyisobutylene–polystyrene triblock polymers: Synthesis, characterization, and physical properties. J. Polym. Sci. Part Polym. Chem. 1991, 29, 427–435. [Google Scholar] [CrossRef]
- Gyor, M.; Wang, H.C.; Faust, R. Living carbocationic polymerization of isobutylene with blocked bifunctional initiators in the presence of di-tert-butylpyridine as a proton trap. J. Macromol. Sci. Part A Pure Appl. Chem. 1992, A29, 639–653. [Google Scholar] [CrossRef]
- Fodor, Z.; Gyor, M.; Wang, H.C.; Faust, R. Living carbocationic polymerization of styrene in the presence of proton trap. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, A30, 349–363. [Google Scholar] [CrossRef]
- Gyor, M.; Fodor, Z.; Wang, H.C.; Faust, R. Polyisobutylene-based thermoplastic elastomers. I. Synthesis and characterization of polystyrene-polyisobutylene-polystyrene triblock copolymers. J. Macromol. Sci. Part A Pure Appl. Chem. 1994, A31, 2055–2056. [Google Scholar] [CrossRef]
- Storey, R.F.; Chisholm, B.J.; Masse, M.A. Morphology and physical properties of poly(styrene-b-isobutylene-b-styrene) block copolymers. Polymer 1996, 37, 2925–2938. [Google Scholar] [CrossRef]
- Storey, R.F.; Chisholm, B.J. Aspects of the synthesis of poly(styrene-b-isobutylene-b-styrene) block copolymers using living carbocationic polymerization. Macromolecules 1993, 26, 6727–6733. [Google Scholar] [CrossRef]
- Cao, X.; Faust, R. Polyisobutylene-based thermoplastic elastomers. 5. Poly(styrene-b-isobutylene-b-styrene) triblock copolymers by coupling of living poly(styrene-b-isobutylene) diblock copolymers. Macromolecules 1999, 32, 5487–5494. [Google Scholar] [CrossRef]
- Puskas, J.E.; Luo, W. Slow initiation in living polymerization with reversible end capping: Kinetic analysis of the initiation in carbocationic styrene polymerization with 2-chloro-2,4,4-trimethylpentane/TiCl4. Macromolecules 2003, 36, 6942–6944. [Google Scholar] [CrossRef]
- Kim, M.S.; Faust, R. Penultimate effect in the initiation of the cationic polymerization of isobutylene: Kinetic study of initiation with (3-chloro-1,1,3-trimethylbutyl)benzene. Macromolecules 2002, 35, 5320–5322. [Google Scholar] [CrossRef]
- Storey, R.F.; Curry, C.L.; Brister, B. Carbocationic rearrangement in controlled/living isobutylene polymerization. Macromolecules 1998, 31, 1058–1063. [Google Scholar] [CrossRef]
- Yan, P.-F.; Guo, A.-R.; Liu, Q.; Wu, Y.-X. Living cationic polymerization of isobutylene coinitiated by FeCl3 in the presence of isopropanol. Polym. Sci. Part A Polym. Chem. 2012, 50, 3383–3392. [Google Scholar] [CrossRef]
- Storey, R.F.; Chisholm, B.J.; Choate, K.R. Synthesis and characterization of PS-PIB-PS triblock copolymers. J. Macromol. Sci. Appl. Chem. 1994, 31, 969–987. [Google Scholar] [CrossRef]
- Fodor, Z.; Faust, R. Polyisobutylene-based thermoplastic elastomers. IV. Synthesis of poly(styrene-block-isobutylene-block-styrene) triblock copolymers using n-butyl chloride as solvent. J. Macromol. Sci. Part Pure Appl. Chem. 1996, 33, 305–324. [Google Scholar] [CrossRef]
- Everland, H.; Kops, J.; Nielsen, A.; Iván, B. Living carbocationic polymerization of isobutylene and synthesis of ABA block copolymers by conventional laboratory techniques. Polym. Bull. 1993, 31, 159–166. [Google Scholar] [CrossRef]
- Storey, R.F.; Lee, Y. Living carbocationic polymerization of isobutylene using blocked dicumyl chloride or tricumyl chloride/TiCl4/pyridine initiating system. J. Macromol. Sci. Appl. Chem. 1992, 29, 1017–1030. [Google Scholar] [CrossRef]
- Hadjikyriacou, S.; Acar, M.; Faust, R. Living and controlled polymerization of isobutylene with alkylaluminum halides as coinitiators. Macromolecules 2004, 37, 7543–7547. [Google Scholar] [CrossRef]
- Storey, R.F.; Choate, K.R. Kinetic investigation of the living cationic polymerization of isobutylene using a t-Bu-m-DCC/TiCl4/2,4-DMP initiating system. Macromolecules 1997, 30, 4799–4806. [Google Scholar] [CrossRef]
- Storey, R.F.; Donnalley, A.B. TiCl4 reaction order in living isobutylene polymerization at low [TiCl4]:[chain end] ratios. Macromolecules 2000, 33, 53–59. [Google Scholar] [CrossRef]
- Bae, Y.C.; Faust, R. β-Proton elimination by free bases in the living carbocationic polymerization of isobutylene. Macromolecules 1997, 30, 7341–7344. [Google Scholar] [CrossRef]
- Held, D.; Ivan, B.; Muller, A.H.E.; de Jong, F.; Graafland, T. Stability of propagating species in living cationic polymerization of isobutylene. ACS Symp. Ser. 1997, 665, 63–74. [Google Scholar]
- Kostjuk, S.V. Cationic polymerization of p-methoxystyrene co-initiated by B(C6F5)3: Effect of acetonitrile and proton traps. Eur. Polym. J. 2020, 129, 109632. [Google Scholar] [CrossRef]
- Kostjuk, S.V. Recent progress in the Lewis acids co-initiated cationic polymerization of isobutylene and 1,3-dienes. RSC Adv. 2015, 5, 13125–13144. [Google Scholar] [CrossRef]
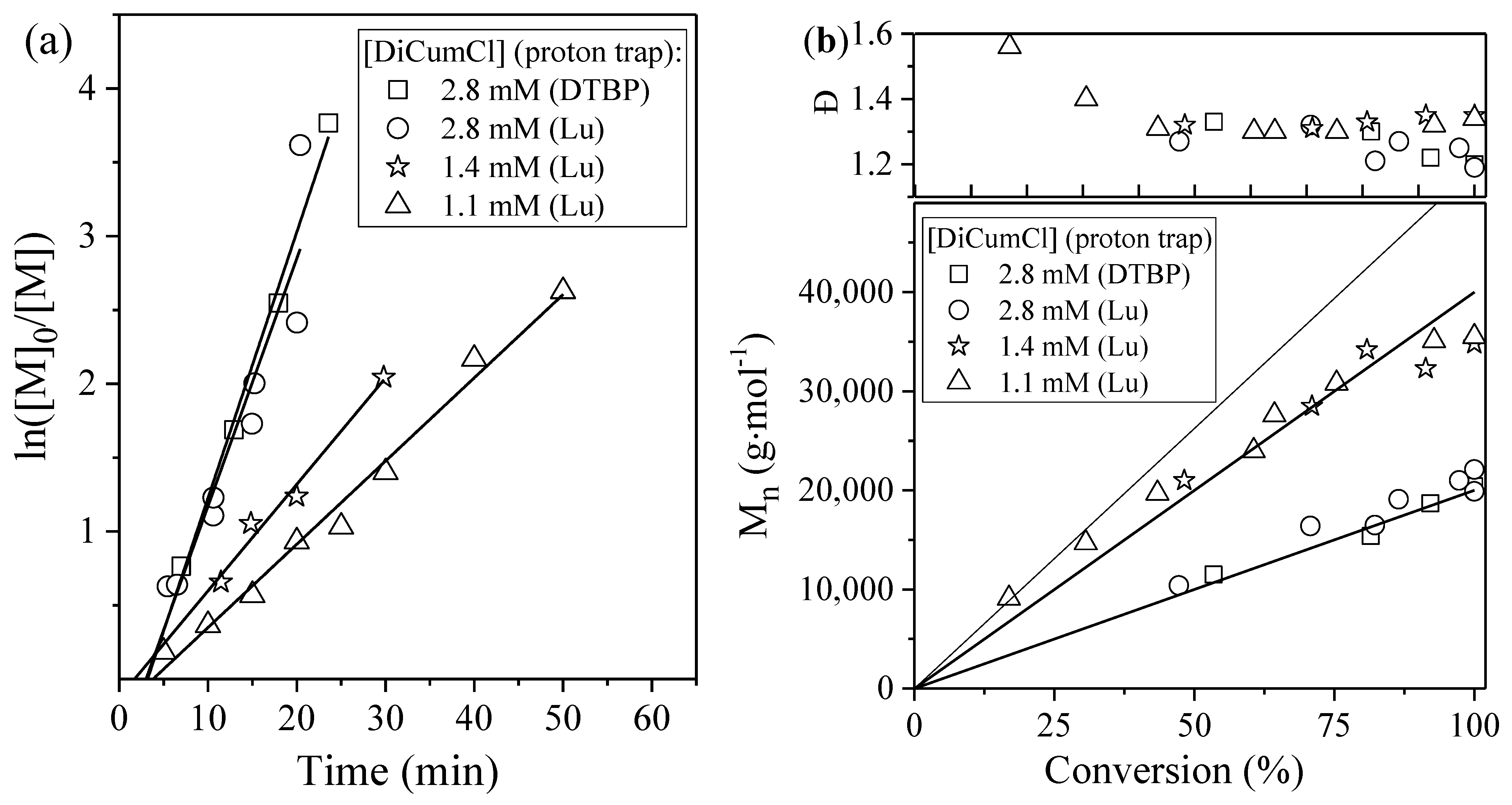
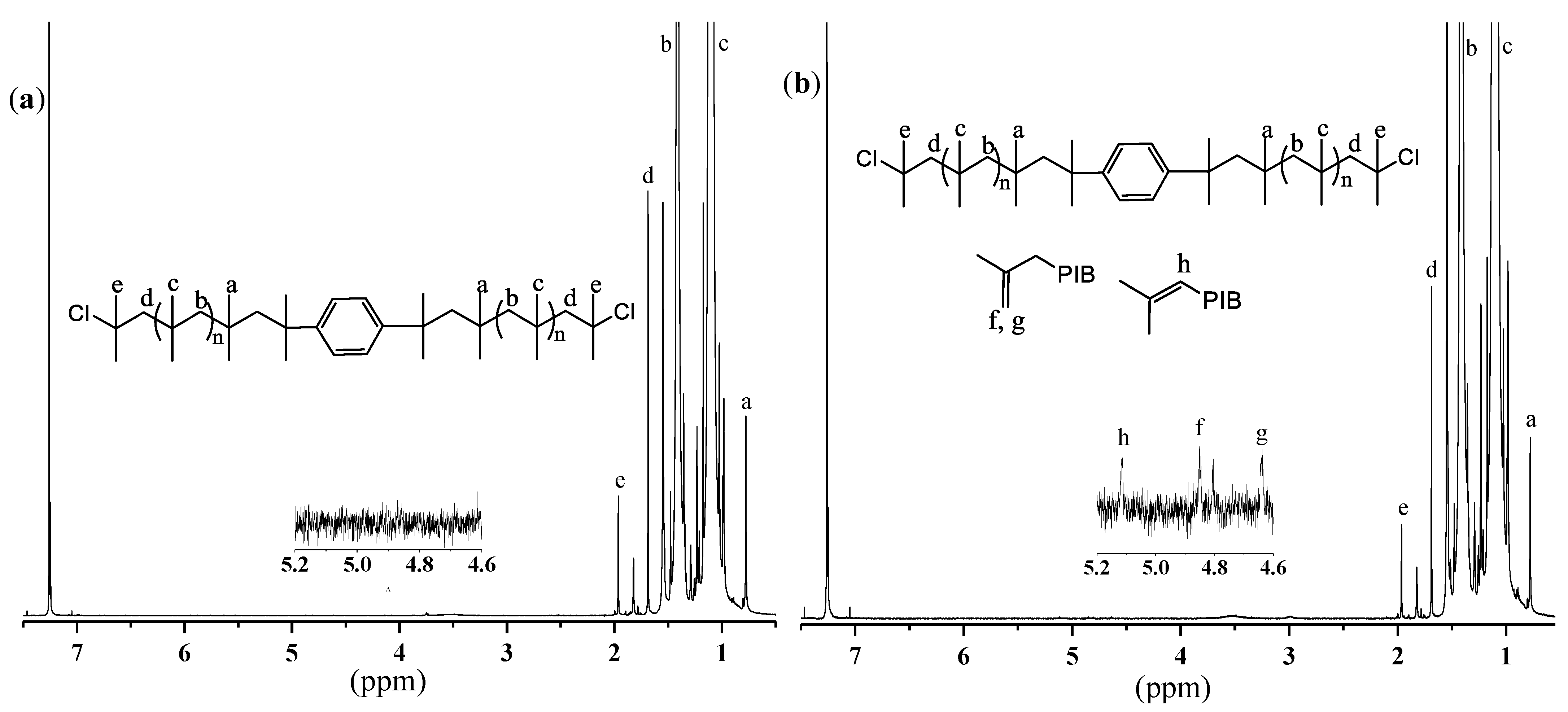

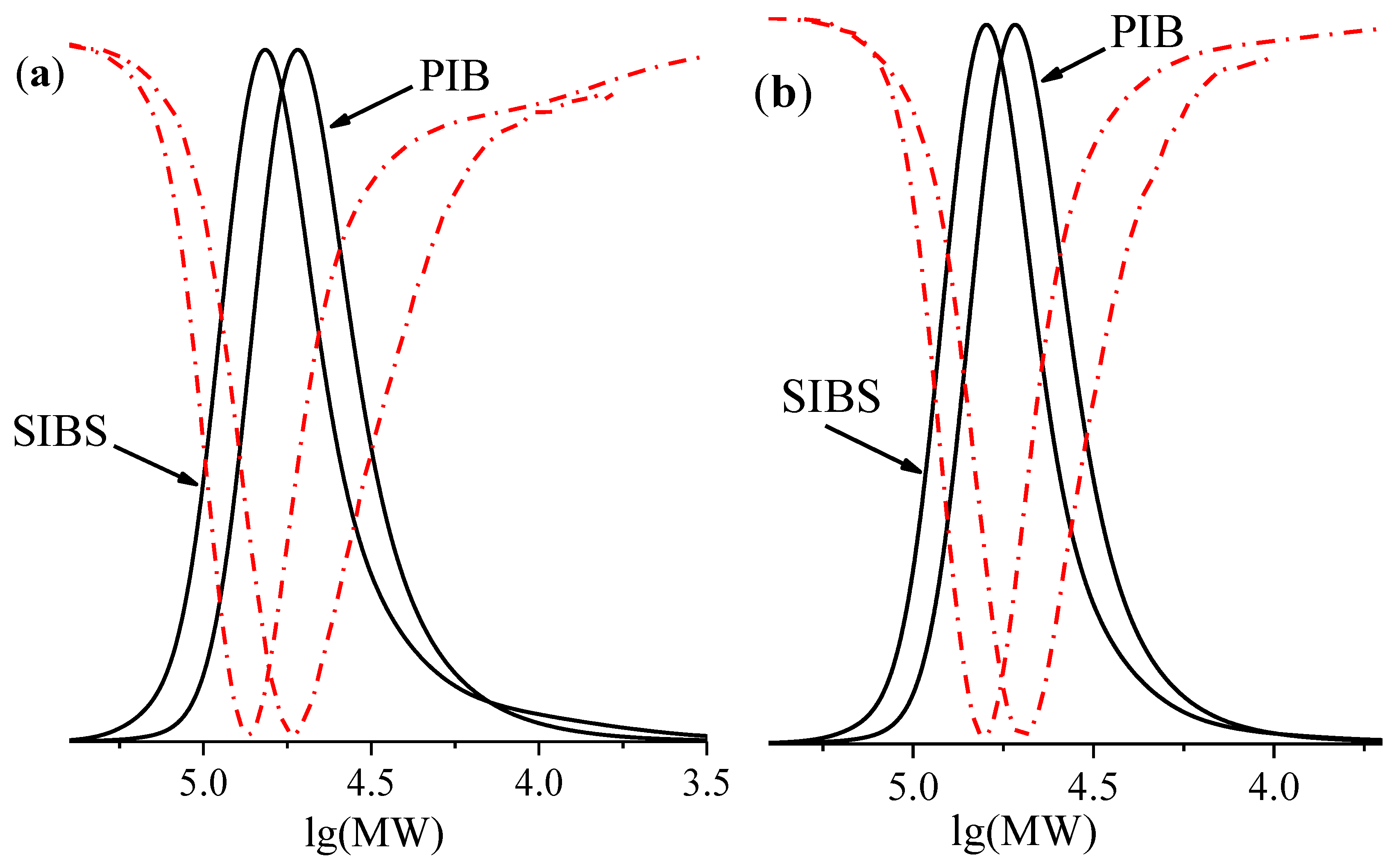

| Run | [DiCumCl] (mM) | [Lu1] 2 (mM) | [Lu2] 2 (mM) | Mn, theor3 (g mol−1) | Mn (PIB) (g mol−1) | Đ | Mn, theor4 (g mol−1) | Mn (SIBS) (g mol−1) | Đ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.8 | 5.8 | 0 | 20,230 | 21,400 | 1.23 | 30,230 | 24,900 | 1.37 |
| 2 | 2.8 | 5.8 | 5.8 | 20,230 | 21,100 | 1.17 | 30,230 | 29,400 | 1.21 |
| 3 | 1.4 | 0 | 0 | 40,230 | 43,000 | 1.43 | 60,230 | 25,000 | 2.62 |
| 4 | 1.4 | 5.8 | 0 | 40,230 | 40,500 | 1.33 | 60,230 | 33,200 | 1.86 |
| 5 | 1.4 | 11.7 | 0 | 40,230 | 41,000 | 1.25 | 60,230 | 43,200 | 1.46 |
| 6 | 1.4 | 17.5 | 0 | 40,230 | 45,100 | 1.38 | 60,230 | 42,700 | 1.46 |
| 7 | 1.4 | 11.7 | 5.8 | 40,230 | 42,000 | 1.18 | 60,230 | 49,700 | 1.23 |
| 8 | 1.4 | 11.7 | 11.7 | 40,230 | 39,600 | 1.19 | 60,230 | 45,800 | 1.21 |
| 9 5 | 1.1 | 5.8 | 5.8 | 52,730 | 41,300 | 1.27 | 75,230 | 59,300 | 1.62 |
| 10 5 | 1.1 | 11.7 | 11.7 | 52,730 | 51,800 | 1.21 | 75,230 | 64,200 | 1.28 |
| Run | Mn (SIBS) (g mol−1) | Đ | Mn(PSt) 1 (g mol−1) | St 2 (wt%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|
| 1 | 33,000 | 1.30 | 6000 | 29 | 3.58 | 240 |
| 2 | 47,200 | 1.25 | 5700 | 29 | 6.93 | 800 |
| 3 | 54,800 | 1.40 | 4600 | 27 | 6.07 | 1140 |
| 4 | 64,200 | 1.28 | 6200 | 25 | 13.56 | 1180 |
| 5 | 59,300 | 1.62 | 9000 | 29 | 12.57 | 1470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarevich, M.I.; Nikishau, P.A.; Berezianko, I.A.; Glushkova, T.V.; Rezvova, M.A.; Ovcharenko, E.A.; Bekmukhamedov, G.E.; Yakhvarov, D.G.; Kostjuk, S.V. Aspects of the Synthesis of Poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated Cationic Polymerization in Open Conditions. Macromol 2021, 1, 243-255. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1040017
Makarevich MI, Nikishau PA, Berezianko IA, Glushkova TV, Rezvova MA, Ovcharenko EA, Bekmukhamedov GE, Yakhvarov DG, Kostjuk SV. Aspects of the Synthesis of Poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated Cationic Polymerization in Open Conditions. Macromol. 2021; 1(4):243-255. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1040017
Chicago/Turabian StyleMakarevich, Miraslau I., Pavel A. Nikishau, Ivan A. Berezianko, Tatiana V. Glushkova, Maria A. Rezvova, Evgeny A. Ovcharenko, Giyjaz E. Bekmukhamedov, Dmitry G. Yakhvarov, and Sergei V. Kostjuk. 2021. "Aspects of the Synthesis of Poly(styrene-block-isobutylene-block-styrene) by TiCl4-Co-initiated Cationic Polymerization in Open Conditions" Macromol 1, no. 4: 243-255. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1040017







