On the Shoulders of a Giant: Contributions of Thomas Grogan, MD to Hematopathology
Abstract
:1. Introduction
2. Arrival at Stanford and Entry into Hematopathology
3. Beginnings of Immunohistochemistry
4. The Promise of Immunohistochemistry
5. Realizing a Vision
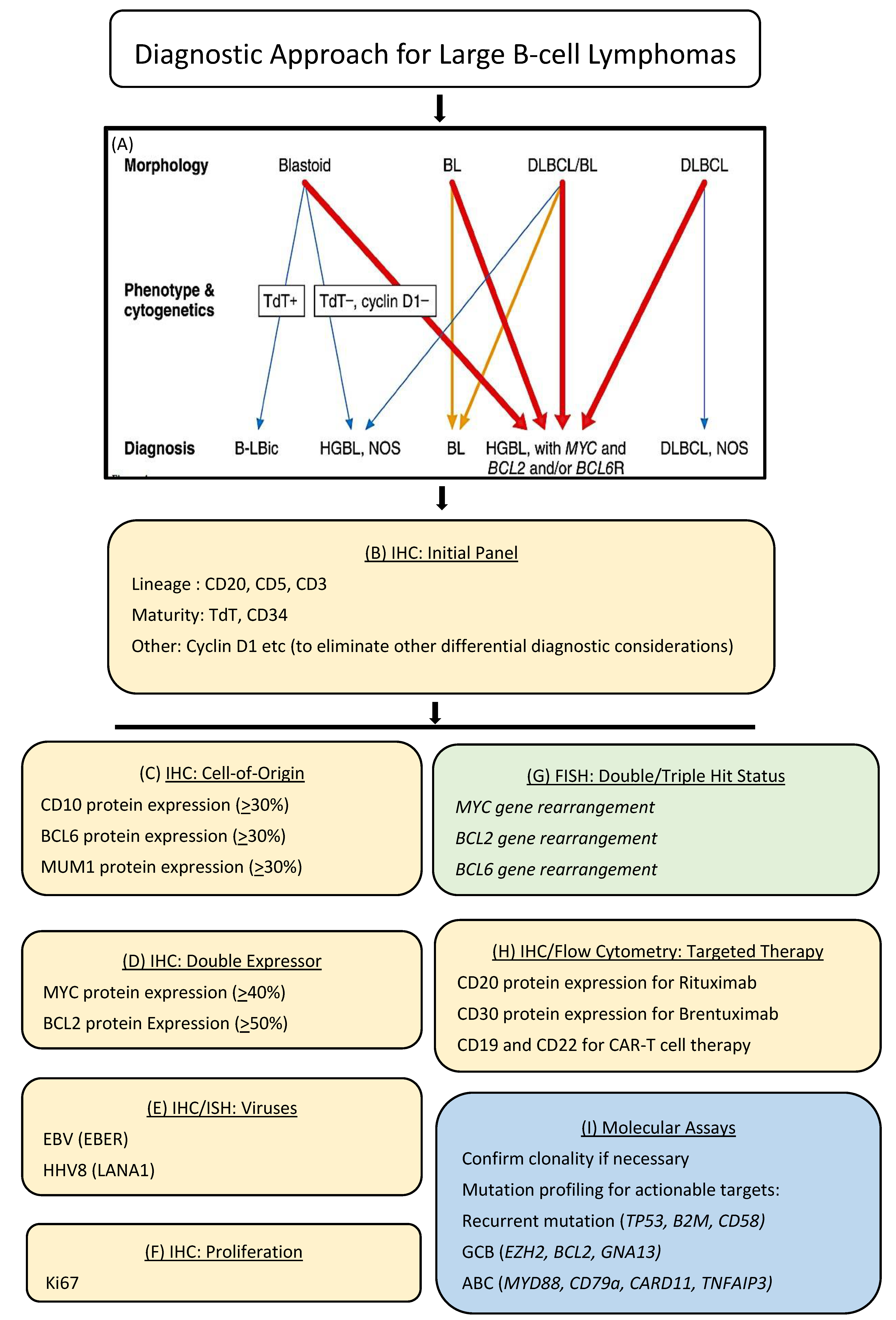
6. Enabling Precision Health
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Grogan, T. Chasing the Invisible: A Doctor’s Quest to Abolish the Last Unseen Cancer Cell. Koehlerbooks: Virginia Beach, VA, USA, 2019; ISBN 978-1-63393-941-7. [Google Scholar]
- Levy, R.; Warnke, R.; Dorfman, R.F.; Haimovich, J. The monoclonality of human B-cell lymphomas. J. Exp. Med. 1977, 145, 1014–1028. [Google Scholar] [CrossRef] [Green Version]
- Warnke, R.; Levy, R. Immunopathology of follicular lymphomas. A model of B-lymphocyte homing. N. Engl. J. Med. 1978, 298, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Warnke, R.; Pederson, M.; Williams, C.; Levy, R. A study of lymphoproliferative diseases comparing immunofluorescence with immunohistochemistry. Am. J. Clin. Pathol. 1978, 70, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, C.D. Henry Kaplan and the Story of Hodgkin’s Disease. Stanford University Press: Stanford, CA, USA, 2010; ISBN 978-0-8047-6866-5. [Google Scholar]
- Society for Hematopathology. Available online: https://www.society-for-hematopathology.org/web/about-history.php (accessed on 26 February 2021).
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Warnke, R.; Levy, R. Detection of T and B cell antigens hybridoma monoclonal antibodies: A biotin-avidin-horseradish peroxi-dase method. J. Histochem. Cytochem. 1980, 28, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engleman, E.G.; Warnke, R.; Fox, R.I.; Dilley, J.; Benike, C.J.; Levy, R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 1981, 78, 1791–1795. [Google Scholar] [CrossRef] [Green Version]
- Link, M.; Warnke, R.; Finlay, J.; Amylon, M.; Miller, R.; Dilley, J.; Levy, R. A single monoclonal antibody identifies T-cell lineage of childhood lymphoid malignancies. Blood 1983, 62, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Grogan, T.M.; Warnke, R.A.; Kaplan, H.S. A comparative study of Burkitt’s and non-Burkitt’s “undifferentiated” malignant lym-phoma: Immunologic, cytochemical, ultrastructural, cytologic, histopathologic, clinical and cell culture features. Cancer 1982, 49, 1817–1828. [Google Scholar] [CrossRef]
- Warnke, R.; Miller, R.; Grogan, T.; Pederson, M.; Dilley, J.; Levy, R. Immunologic phenotype in 30 patients with diffuse large-cell lymphoma. N. Engl. J. Med. 1980, 303, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bindl, J.M.; Warnke, R.A. Advantages of detecting monoclonal antibody binding to tissue sections with biotin and avidin rea-gents in Coplin jars. Am. J. Clin. Pathol. 1986, 85, 490–493. [Google Scholar] [CrossRef]
- Colby, T.V.; Warnke, R.A.; Burke, J.S.; Dorfman, R.F. Differentiation of chronic lymphocytic leukemia from Hodgkin’s disease using immunologic marker studies. Am. J. Surg. Pathol. 1981, 7, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Warnke, R.A.; Gatter, K.C.; Falini, B.; Hildreth, P.; Woolston, R.-E.; Pulford, K.; Cordell, J.L.; Cohen, B.; De Wolf-Peeters, C.; Mason, D.Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N. Engl. J. Med. 1983, 309, 1275–1281. [Google Scholar] [CrossRef]
- Grogan, T.M.; Jolley, C.S.; Rangel, C.S. Immunoarchitecture of the human spleen. Lymphology 1983, 16, 72–82. [Google Scholar] [PubMed]
- Wirt, D.P.; Grogan, T.M.; Nagle, R.B.; Copeland, J.G.; Richter, L.C.; Rangel, C.S.; Schuchardt, M.; Fosse, J.; Layton, J.M. A comprehensive immunotopographic map of human thymus. J. Histochem. Cytochem. 1988, 36, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Grogan, T.M.; Tubbs, R.R. A double-blind comparative immunotypic study between two institutions phenotyping non-hodgkin’s lymphomas. Am. J. Clin. Pathol. 1987, 87, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Grogan, T.M.; Fielder, K.; Rangel, C.; Jolley, C.J.; Wirt, D.P.; Hicks, M.J.; Miller, T.P.; Brooks, R.; Greenberg, B.; Jones, S. Peripheral T-cell lymphoma: Aggressive disease with heterogeneous immunotypes. Am. J. Clin. Pathol. 1985, 83, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronland, R.; Grogan, T.; Spier, C.; Wirt, D.; Rangel, C.; Richter, L.; Durie, B.; Greenberg, B.; Miller, T.; Jones, S. Immunotopographic assessment of lymphoid and plasma cell malignancies in the bone marrow. Hum. Pathol. 1985, 16, 1247–1254. [Google Scholar] [CrossRef]
- Lippman, S.M.; Volk, J.R.; Spier, C.M.; Grogan, T.M. Clonal ambiguity of human immunodeficiency virus-associated lymphomas. Similarity to posttransplant lymphomas. Arch. Pathol. Lab. Med. 1988, 112, 128–132. [Google Scholar] [PubMed]
- Grogan, T.M.; Lippman, S.M.; Spier, C.M.; Slymen, D.J.; Rybski, J.A.; Rangel, C.S.; Richter, L.C.; Miller, T.P. Independent prognostic signif-icance of a nuclear proliferation antigen in diffuse large cell lymphomas as determined by the monoclonal antibody Ki-67. Blood 1988, 71, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.P.; Lippman, S.M.; Spier, C.M.; Slymen, D.J.; Grogan, T.M. HLA-DR (Ia) immune phenotype predicts outcome for patients with diffuse large cell lymphoma. J. Clin. Investig. 1988, 82, 370–372. [Google Scholar] [CrossRef] [Green Version]
- Lippman, S.M.; Miller, T.P.; Spier, C.M.; Slymen, D.J.; Grogan, T.M. The prognostic significance of the immunotype in diffuse large-cell lymphoma: A comparative study of the T-cell and B-cell phenotype. Blood 1988, 72, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Dalton, W.S.; Grogan, T.M.; Rybski, J.A.; Scheper, R.J.; Richter, L.; Kailey, J.; Broxterman, H.J.; Pinedo, H.M.; Salmon, S.E. Immunohisto-chemical detection and quantitation of P-glycoprotein in multiple drug-resistant human myeloma cells: Association with level of drug resistance and drug accumulation. Blood 1989, 73, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Dalton, W.S.; Grogan, T.M.; Meltzer, P.S.; Scheper, R.J.; Durie, B.G.; Taylor, C.W.; Miller, T.P.; Salmon, S.E. Drug-resistance in multiple myeloma and non-Hodgkin’s lymphoma: Detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J. Clin. Oncol. 1989, 7, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Warnke, R.A.; Pulford, K.A.; Pallesen, G.; Ralfkiaer, E.; Brown, D.C.; Gatter, K.C.; Mason, D.Y. Diagnosis of myelomonocytic and macrophage neoplasms in routinely processed tissue biopsies with monoclonal antibody KP1. Am. J. Pathol. 1989, 135, 1089–1095. [Google Scholar] [PubMed]
- Pileri, S.A.; Grogan, T.M.; Harris, N.L.; Banks, P.; Campo, E.; Chan, J.K.; Favera, R.D.; Delsol, G.; De Wolf-Peeters, C.; Falini, B.; et al. Tumours of histiocytes and accessory dendritic cells: An immunohisto-chemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 2002, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.L.; Jaffe, E.S.; Stein, H.; Banks, P.M.; Chan, J.K.; Cleary, M.L.; Delsol, G.; De Wolf-Peeters, C.; Falini, B.; Gatter, K.C.; et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84, 1361–1392. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, E.; Harris, N.; Stein, H.; Vardiman, J.W. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2001. [Google Scholar]
- Swerdlow, S.; Campo, E.; Harris, N.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC Press: Lyon, France, 2008. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th Ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Maloney, D.G.; Liles, T.M.; Czerwinski, D.K.; Waldichuk, C.; Rosenberg, J.; Grillo-Lopez, A.; Levy, R. Phase I clinical trial using esca-lating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lym-phoma. Blood 1994, 84, 2457–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloney, D.G.; Grillo-López, A.J.; White, C.A.; Bodkin, D.; Schilder, R.J.; Neidhart, J.A.; Janakiraman, N.; Foon, K.A.; Liles, T.M.; Dallaire, B.K.; et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997, 90, 2188–2195. [Google Scholar] [CrossRef]
- Davis, T.A.; Grillo-López, A.J.; White, C.A.; McLaughlin, P.; Czuczman, M.S.; Link, B.K.; Maloney, D.G.; Weaver, R.L.; Rosenberg, J.; Levy, R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: Safety and efficacy of re-treatment. J. Clin. Oncol. 2000, 18, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Neste, E.V.D.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar]
- Rimsza, L.M.; Leblanc, M.L.; Unger, J.M.; Miller, T.P.; Grogan, T.M.; Persky, D.O.; Martel, R.R.; Sabalos, C.M.; Seligmann, B.; Braziel, R.M.; et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood 2008, 112, 3425–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.M.; Thalji, N.M.; Greenberg, A.J.; Tapia, C.J.; Windebank, A.J. Rituximab for non-Hodgkin’s lymphoma: A story of rapid success in translation. Clin. Transl. Sci. 2014, 7, 82–86. [Google Scholar] [CrossRef]
- Levy, R. A perspective on monoclonal antibody therapy: Where we have been and where we are going. Semin. Hematol. 2000, 37, 43–46. [Google Scholar] [CrossRef]
- Ansell, S.M.; Horwitz, S.M.; Engert, A.; Khan, K.D.; Lin, T.; Strair, R.; Keler, T.; Graziano, R.; Blanset, D.; Yellin, M.; et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J. Clin. Oncol. 2007, 25, 2764–2769. [Google Scholar] [CrossRef]
- Forero-Torres, A.; Leonard, J.P.; Younes, A.; Rosenblatt, J.D.; Brice, P.; Bartlett, N.L.; Bosly, A.; Pinter-Brown, L.; Kennedy, D.; Sievers, E.L.; et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 2009, 146, 171–179. [Google Scholar] [CrossRef]
- Fanale, M.A.; Forero-Torres, A.; Rosenblatt, J.D.; Advani, R.H.; Franklin, A.R.; Kennedy, D.A.; Han, T.H.; Sievers, E.L.; Bartlett, N.L. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin. Cancer Res. 2012, 18, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Makita, S.; Maruyama, D.; Tobinai, K. Safety and Efficacy of Brentuximab Vedotin in the Treatment of Classic Hodgkin Lym-phoma. Onco Targets Ther. 2020, 13, 5993–6009. [Google Scholar] [CrossRef]
- Sabattini, E.; Pizzi, M.; Tabanelli, V.; Baldin, P.; Sacchetti, C.S.; Agostinelli, C.; Zinzani, P.L.; Pileri, S.A. CD30 expression in peripheral T-cell lymphomas. Haematologica 2013, 98, e81–e82. [Google Scholar] [CrossRef]
- Bossard, C.; Dobay, M.P.; Parrens, M.; Lamant, L.; Missiaglia, E.; Haioun, C.; Martin, A.; Fabiani, B.; Delarue, R.; Tournilhac, O.; et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: High correlation with mRNA levels. Blood 2014, 124, 2983–2986. [Google Scholar] [CrossRef] [Green Version]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Roemer, M.G.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Torlakovic, E.; Lim, H.J.; Adam, J.; Barnes, P.; Bigras, G.; Chan, A.W.H.; Cheung, C.C.; Chung, J.H.; Couture, C.; Fiset, P.O.; et al. “Interchangeability” of PD-L1 immunohisto-chemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 2020, 33, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.; Bruno, B.; Neelapu, S. Editorial: CAR T-Cell Therapies in Hematologic Tumors. Front. Oncol. 2020, 10, 588134. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, A.B.; Dave, S.S. Clinical Applications of the Genomic Landscape of Aggressive Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, E.; Khalili, P.; Beuhler, K.; Siddiqi, I.; Vasef, M.A. BRAF V600E Mutation Across Multiple Tumor Types: Correlation between DNA-based Sequencing and Mutation-specific Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.D.; Mendez-Lago, M.; Mungall, A.J.; Goya, R.; Mungall, K.L.; Corbett, R.D.; Johnson, N.A.; Severson, T.M.; Chiu, R.; Field, M.; et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011, 476, 298–303. [Google Scholar] [CrossRef]
- Mohammad, F.; Weissmann, S.; Leblanc, B.; Pandey, D.P.; Højfeldt, J.W.; Comet, I.; Zheng, C.; Johansen, J.V.; Rapin, N.; Porse, N.R.B.T.; et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 2017, 23, 483–492. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Huang, S.; Nong, L.; Li, D.; Zhang, B.; Li, T. Aberrant differential expression of EZH2 and H3K27me3 in extranodal NK/T-cell lymphoma, nasal type, is associated with disease progression and prognosis. Hum. Pathol. 2019, 83, 166–176. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natkunam, Y.; Warnke, R.A. On the Shoulders of a Giant: Contributions of Thomas Grogan, MD to Hematopathology. Hemato 2021, 2, 103-115. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010006
Natkunam Y, Warnke RA. On the Shoulders of a Giant: Contributions of Thomas Grogan, MD to Hematopathology. Hemato. 2021; 2(1):103-115. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010006
Chicago/Turabian StyleNatkunam, Yasodha, and Roger A. Warnke. 2021. "On the Shoulders of a Giant: Contributions of Thomas Grogan, MD to Hematopathology" Hemato 2, no. 1: 103-115. https://0-doi-org.brum.beds.ac.uk/10.3390/hemato2010006




