Nucleoredoxin Downregulation Reduces β-Catenin Levels and Shifts Hematopoietic Differentiation towards Myeloid Lineage In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Knockdown of Nucleoredoxin
2.2. Cell Culture
2.3. Assessment of Cell Proliferation
2.4. Study of Cell Adhesion
2.5. Flow Cytometry
2.6. Western Blot
2.7. Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Effect of NRX Downregulation on Megakaryocytic Differentiation of HEL Cells
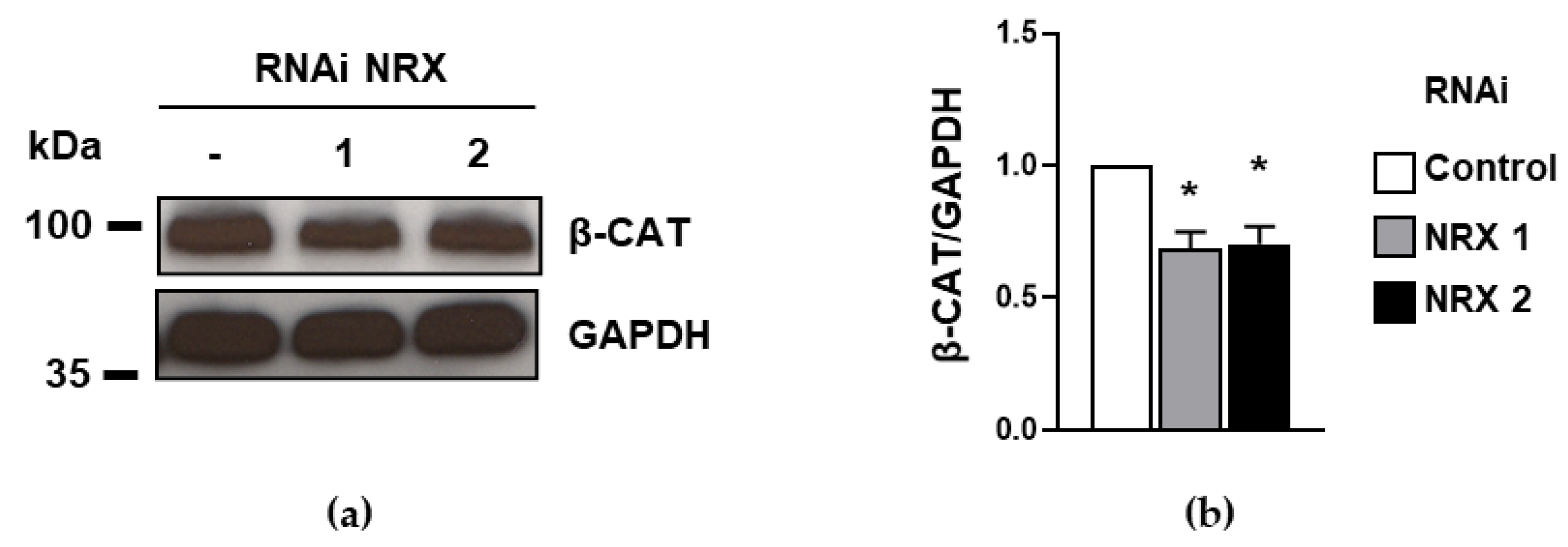
3.2. The Downregulation of NRX Prompts a Decrease in β-Catenin Levels in HEL Cells
3.3. Nrx Downregulation Dramatically Decreases Murine Hematopoietic Progenitors In Vitro
3.4. Surface Markers of Mature Blood Lineages Are Altered in Nrx-Silenced Mouse Hematopoietic Progenitors In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An Evolving Paradigm for Stem Cell Biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Herguido, C.; Guiu, J.; D’Altri, T.; Ingles-Esteve, J.; Dzierzak, E.; Espinosa, L.; Bigas, A. Hematopoietic Stem Cell Development Requires Transient Wnt/β-Catenin Activity. J. Exp. Med. 2012, 209, 1457–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luis, T.C.; Naber, B.A.E.; Roozen, P.P.C.; Brugman, M.H.; De Haas, E.F.E.; Ghazvini, M.; Fibbe, W.E.; Van Dongen, J.J.M.; Fodde, R.; Staal, F.J.T. Canonical Wnt Signaling Regulates Hematopoiesis in a Dosage-Dependent Fashion. Cell Stem Cell 2011, 9, 345–356. [Google Scholar] [CrossRef] [Green Version]
- López-Ruano, G.; Prieto-Bermejo, R.; Ramos, T.L.; San-Segundo, L.; Sánchez-Abarca, L.I.; Sánchez-Guijo, F.; Pérez-Simón, J.A.; Sánchez-Yagüe, J.; Llanillo, M.; Hernández-Hernández, Á.; et al. PTPN13 and β-Catenin Regulate the Quiescence of Hematopoietic Stem Cells and Their Interaction with the Bone Marrow Niche. Stem Cell Rep. 2015, 5, 516–531. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Bermejo, R.; Romo-González, M.; Pérez-Fernández, A.; Ijurko, C.; Hernández-Hernández, Á. Reactive Oxygen Species in Haematopoiesis: Leukaemic Cells Take a Walk on the Wild Side. J. Exp. Clin. Cancer Res. 2018, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardina, J.L.; López-Ruano, G.; Sánchez-Abarca, L.I.; Pérez-Simón, J.A.; Gaztelumendi, A.; Trigueros, C.; Llanillo, M.; Sánchez-Yagüe, J.; Hernández-Hernández, A. P22phox-Dependent NADPH Oxidase Activity Is Required for Megakaryocytic Differentiation. Cell Death Differ. 2010, 17, 1842–1854. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.-H.; Zhang, Z.-Y. Regulatory Mechanisms and Novel Therapeutic Targeting Strategies for Protein Tyrosine Phosphatases. Chem. Rev. 2018, 118, 1069–1091. [Google Scholar] [CrossRef] [Green Version]
- Sardina, J.L.; López-Ruano, G.; Prieto-Bermejo, R.; Sánchez-Sánchez, B.; Pérez-Fernández, A.; Sánchez-Abarca, L.I.; Pérez-Simón, J.A.; Quintales, L.; Sánchez-Yagüe, J.; Llanillo, M.; et al. PTPN13 Regulates Cellular Signalling and β-Catenin Function during Megakaryocytic Differentiation. Biochim. Biophys. Acta - Mol. Cell Res. 2014, 1843, 2886–2899. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, A.; López-Ruano, G.; Prieto-Bermejo, R.; Ijurko, C.; Díez-Campelo, M.; Sánchez-Guijo, F.; Hernández-Hernández, Á. SHP1 and SHP2 Inhibition Enhances the Pro-Differentiative Effect of Phorbol Esters: An Alternative Approach against Acute Myeloid Leukemia. J. Exp. Clin. Cancer Res. 2019, 38, 80. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Michiue, T.; Asashima, M.; Miki, H. The Thioredoxin-Related Redox-Regulating Protein Nucleoredoxin Inhibits Wnt–β-Catenin Signalling through Dishevelled. Nat. Cell Biol. 2006, 8, 501–508. [Google Scholar] [CrossRef]
- Kajla, S.; Mondol, A.S.; Nagasawa, A.; Zhang, Y.; Kato, M.; Matsuno, K.; Yabe-Nishimura, C.; Kamata, T. A Crucial Role for Nox 1 in Redox-Dependent Regulation of Wnt-β-Catenin Signaling. FASEB J. 2012, 26, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Kurooka, H.; Kato, K.; Minoguchi, S.; Takahashi, Y.; Ikeda, J.; Habu, S.; Osawa, N.; Buchberg, A.M.; Moriwaki, K.; Shisa, H.; et al. Cloning and Characterization of the Nucleoredoxin Gene That Encodes a Novel Nuclear Protein Related to Thioredoxin. Genomics 1997, 39, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Miki, H. Nucleoredoxin, a Novel Thioredoxin Family Member Involved in Cell Growth and Differentiation. Antioxid. Redox Signal. 2007, 9, 1035–1057. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Terabayashi, T.; Sakamoto, R.; Okuzaki, D.; Ichise, H.; Nojima, H.; Yoshida, N.; Miki, H. Nucleoredoxin Sustains Wnt/β-Catenin Signaling by Retaining a Pool of Inactive Dishevelled Protein. Curr. Biol. 2010, 20, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Bahn, Y.J.; Lee, K.-P.; Lee, S.-M.; Choi, J.Y.; Seo, Y.-S.; Kwon, K.-S. Nucleoredoxin Promotes Adipogenic Differentiation through Regulation of Wnt/β-Catenin Signaling. J. Lipid Res. 2015, 56, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sanchez, B.; Gutierrez-Herrero, S.; Lopez-Ruano, G.; Prieto-Bermejo, R.; Romo-Gonzalez, M.; Llanillo, M.; Pandiella, A.; Guerrero, C.; Miguel, J.F.S.S.; Sanchez-Guijo, F.; et al. NADPH Oxidases as Therapeutic Targets in Chronic Myelogenous Leukemia. Clin. Cancer Res. 2014, 20, 4014–4025. [Google Scholar] [CrossRef] [Green Version]
- Sardina, J.L.; López-Ruano, G.; Sánchez-Sánchez, B.; Llanillo, M.; Hernández-Hernández, A. Reactive Oxygen Species: Are They Important for Haematopoiesis? Crit. Rev. Oncol. Hematol. 2012, 81, 257–274. [Google Scholar] [CrossRef]
- Jang, Y.Y.; Sharkis, S.J. A Low Level of Reactive Oxygen Species Selects for Primitive Hematopoietic Stem Cells That May Reside in the Low-Oxygenic Niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef] [Green Version]
- Groitl, B.; Jakob, U. Thiol-Based Redox Switches. Biochim. Biophys. Acta 2014, 1844, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Cain, C.J.; Manilay, J.O. Hematopoietic Stem Cell Fate Decisions Are Regulated by Wnt Antagonists: Comparisons and Current Controversies. Exp. Hematol. 2013, 41, 3–16. [Google Scholar] [CrossRef]
- Verbeek, S.; Izon, D.; Hofhuis, F.; Robanus-Maandag, E.; te Riele, H.; van de Wetering, M.; Oosterwegel, M.; Wilson, A.; MacDonald, H.R.; Clevers, H. An HMG-Box-Containing T-Cell Factor Required for Thymocyte Differentiation. Nature 1995, 374, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.C.; Nattamai, K.J.; Dörr, K.; Marka, G.; Uberle, B.; Vas, V.; Eckl, C.; Andrä, I.; Schiemann, M.; Oostendorp, R.A.J.J.; et al. A Canonical to Non-Canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing. Nature 2013, 503, 392–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paluru, P.; Hudock, K.M.; Cheng, X.; Mills, J.A.; Ying, L.; Galvão, A.M.; Lu, L.; Tiyaboonchai, A.; Sim, X.; Sullivan, S.K.; et al. The Negative Impact of Wnt Signaling on Megakaryocyte and Primitive Erythroid Progenitors Derived from Human Embryonic Stem Cells. Stem Cell Res. 2013, 12, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luis, T.C.; Ichii, M.; Brugman, M.H.; Kincade, P.; Staal, F.J.T. Wnt Signaling Strength Regulates Normal Hematopoiesis and Its Deregulation Is Involved in Leukemia Development. Leukemia 2012, 26, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Target | ID | Sequence (5′-3′) |
|---|---|---|
| Human NRX | NRX 1 NRX 2 | AAACAGTACTTCAGTGAGA AGAAAATCATTGCCAAGTA |
| Mouse Nrx | Nrx 1 Nrx 2 | AGATCATTGCCAAGTACAA ATACCGAGTCTCCAACATT |
| Target Gene | Target Strand | Sequence (5′-3′) |
|---|---|---|
| Human NRX | Forward Reverse | ACCCAGAAGGTCTGGAGTTC CCAATGTGCGGAGAAATAGA |
| Mouse Nrx | Forward Reverse | TCGTTAGTGCAGACAGGTCA TGCCTTGGATTCCATACAGT |
| Human ACTB | Forward Reverse | CACCACACCTTCTACAATGA ACATGATCTGGGTCATCTTC |
| Mouse Actb | Forward Reverse | CAGCCTTCCTTCTTGGGTAT TGGCATAGAGGTCTTTACGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Fernández, A.; López-Ruano, G.; Prieto-Bermejo, R.; Sánchez-Bernal, C.; Sánchez-Yagüe, J.; Hernández-Hernández, Á. Nucleoredoxin Downregulation Reduces β-Catenin Levels and Shifts Hematopoietic Differentiation towards Myeloid Lineage In Vitro. BioChem 2021, 1, 26-35. https://0-doi-org.brum.beds.ac.uk/10.3390/biochem1010003
Pérez-Fernández A, López-Ruano G, Prieto-Bermejo R, Sánchez-Bernal C, Sánchez-Yagüe J, Hernández-Hernández Á. Nucleoredoxin Downregulation Reduces β-Catenin Levels and Shifts Hematopoietic Differentiation towards Myeloid Lineage In Vitro. BioChem. 2021; 1(1):26-35. https://0-doi-org.brum.beds.ac.uk/10.3390/biochem1010003
Chicago/Turabian StylePérez-Fernández, Alejandro, Guillermo López-Ruano, Rodrigo Prieto-Bermejo, Carmen Sánchez-Bernal, Jesús Sánchez-Yagüe, and Ángel Hernández-Hernández. 2021. "Nucleoredoxin Downregulation Reduces β-Catenin Levels and Shifts Hematopoietic Differentiation towards Myeloid Lineage In Vitro" BioChem 1, no. 1: 26-35. https://0-doi-org.brum.beds.ac.uk/10.3390/biochem1010003







