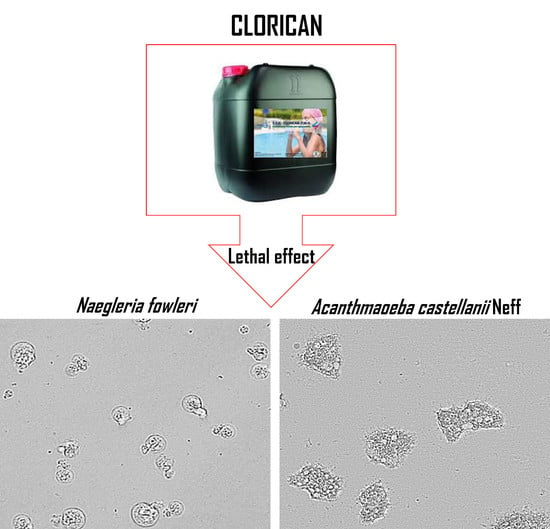
Effect of a Commercial Disinfectant CLORICAN® on Acanthamoeba spp. and Naegleria fowleri Viability
Abstract
:1. Introduction
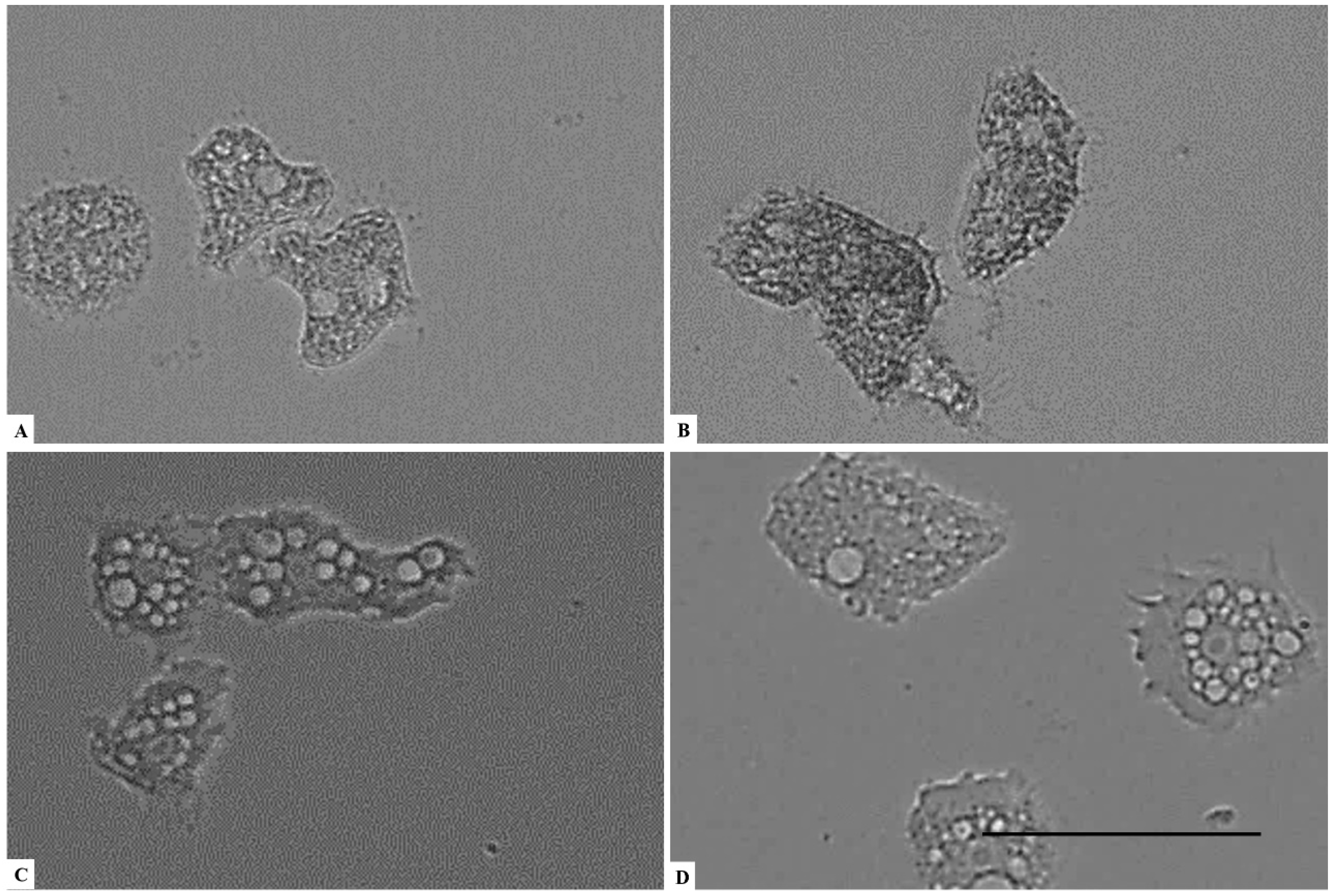
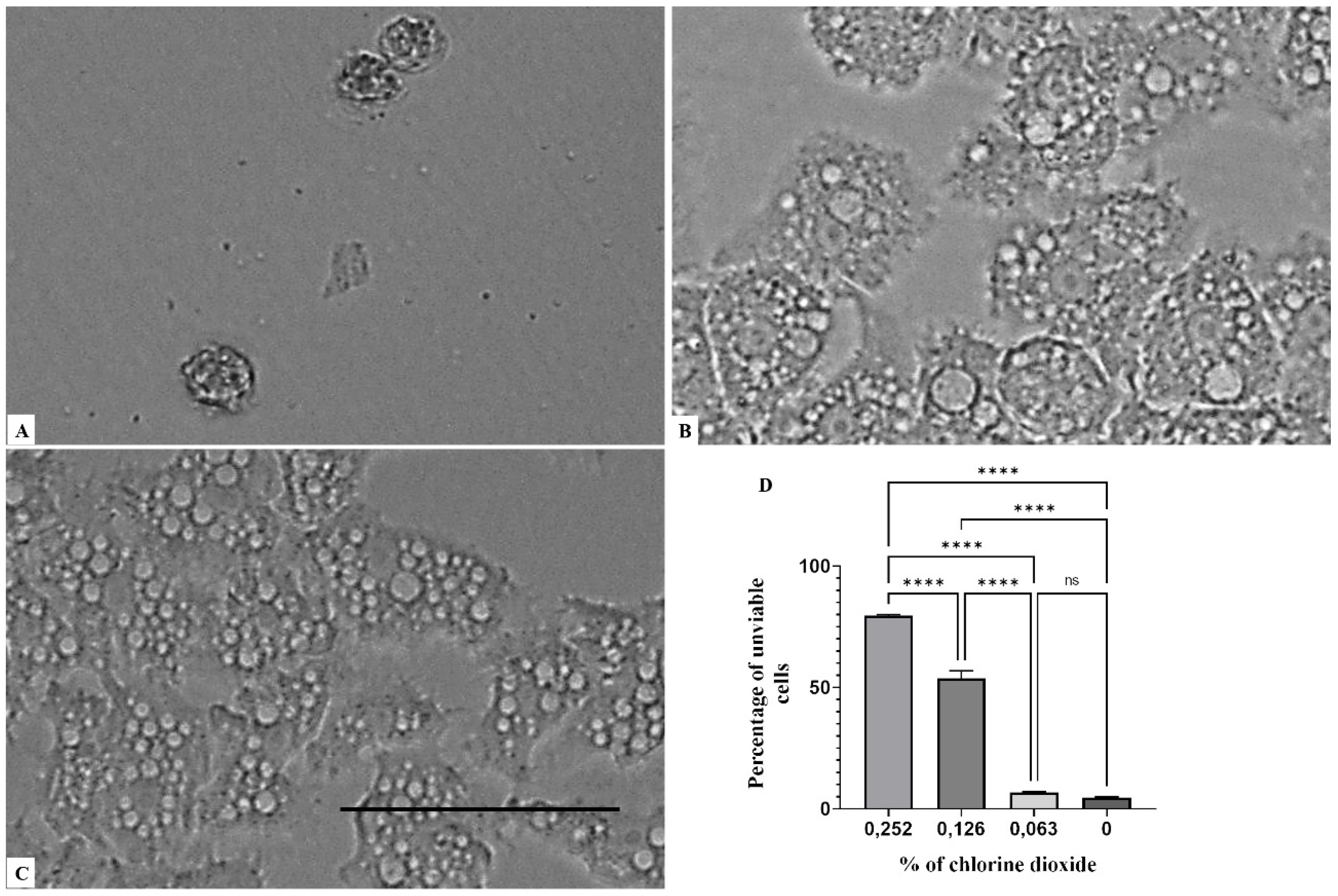
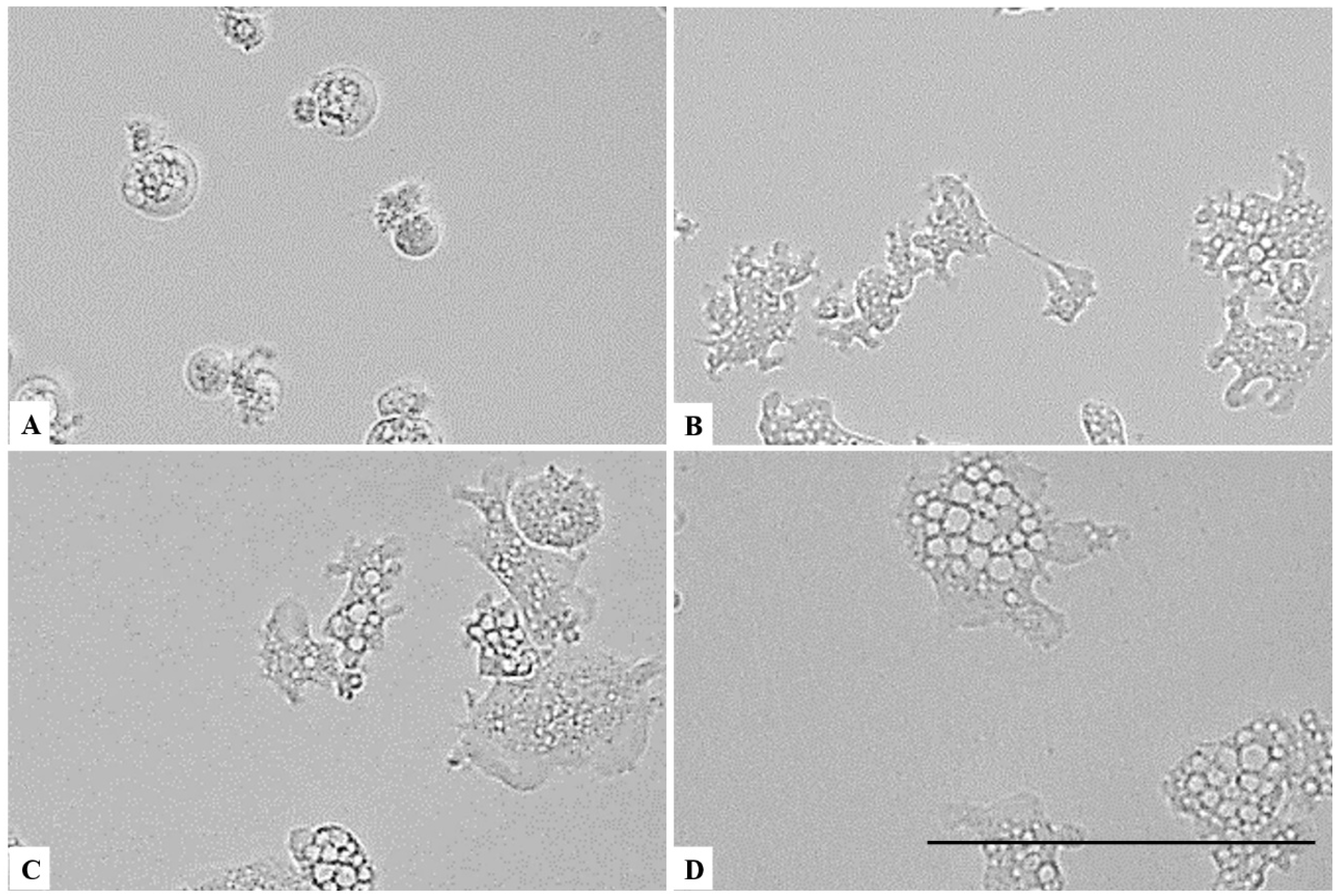
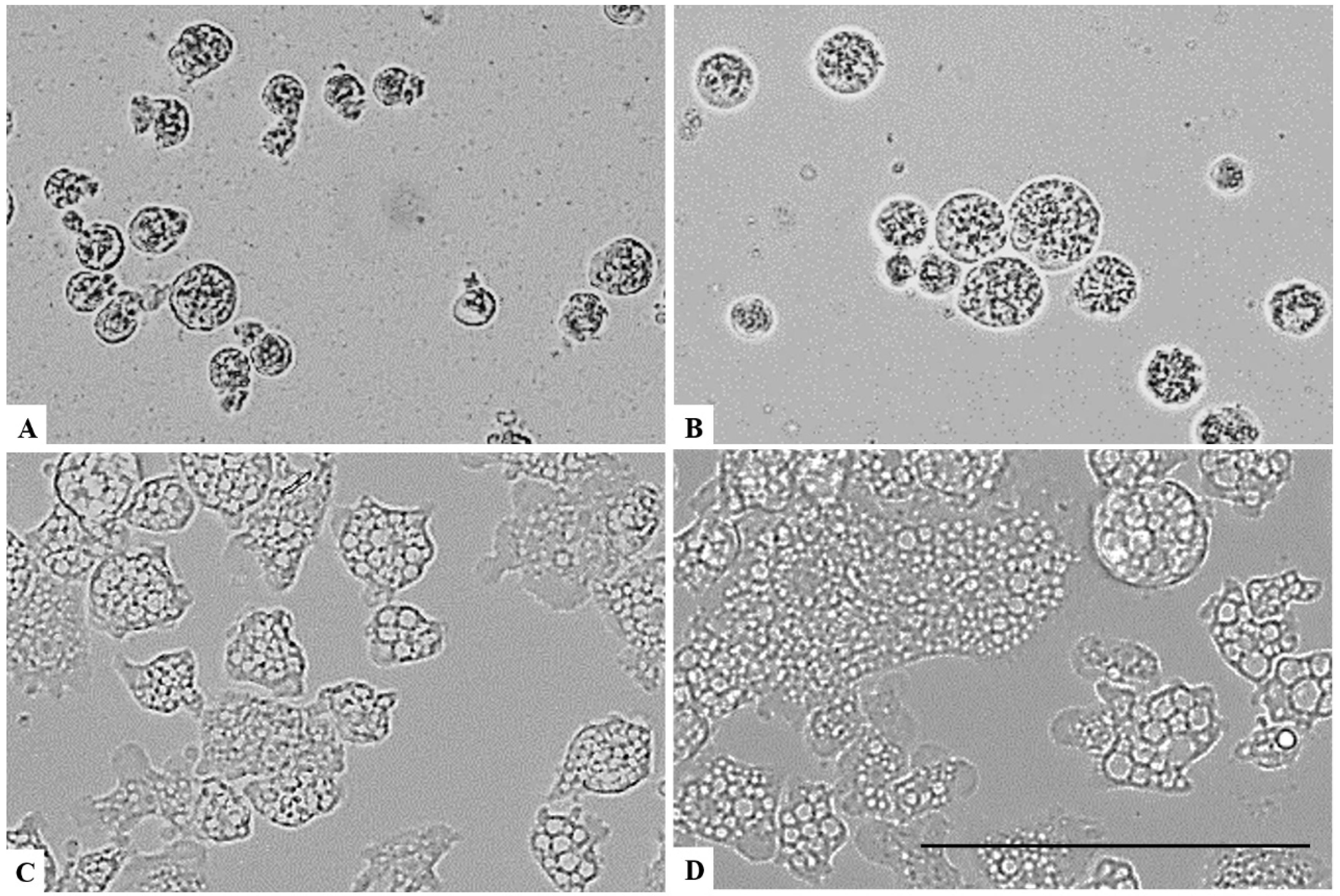
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical
4.2. Cell Culture
4.3. In Vitro Activity Against Trophozoite Stage
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javanmard, E.; Niyyati, M.; Lorenzo-Morales, J.; Lasjerdi, Z.; Behniafar, H.; Mirjalali, H. Molecular identification of waterborne free living amoebae (Acanthamoeba, Naegleria and Vermamoeba) isolated from municipal drinking water and environmental sources, Semnan province, north half of Iran. Exp. Parasitol. 2017, 183, 240–244. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Guidelines for Safe Recreational Water Environments: Volume 2: Swimming Pools and Similar Environments; World Health Organization: Geneva, Switzerland, 2006; p. 118, xiii. [Google Scholar]
- Marciano-Cabral, F.; Puffenbarger, A.R.; Cabral, G.A. The Increasing Importance of Acanthamoeba Infections1. J. Eukaryot. Microbiol. 2000, 47, 29–36. [Google Scholar] [CrossRef]
- Scheid, P.; Zöller, L.; Pressmar, S.; Richard, G.; Michel, R. An extraordinary endocytobiont in Acanthamoeba sp. isolated from a patient with keratitis. Parasitol. Res. 2008, 102, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.; Walochnik, J. An update onAcanthamoebakeratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsaro, D. Update on Acanthamoeba phylogeny. Parasitol. Res. 2020, 119, 3327–3338. [Google Scholar] [CrossRef]
- Maciver, S.K.; Asif, M.; Simmen, M.W.; Lorenzo-Morales, J. A systematic analysis of Acanthamoeba genotype frequency correlated with source and pathogenicity: T4 is confirmed as a pathogen-rich genotype. Eur. J. Protistol. 2013, 49, 217–221. [Google Scholar] [CrossRef]
- Grün, A.-L.; Stemplewitz, B.; Scheid, P. First report of an Acanthamoeba genotype T13 isolate as etiological agent of a keratitis in humans. Parasitol. Res. 2014, 113, 2395–2400. [Google Scholar] [CrossRef]
- Al-Herrawy, A.; Bahgat, M.; Mohammed, A.-E.; Ashour, A.; Hikal, W. Acanthamoeba species in Swimming Pools of Cairo, Egypt. Iran. J. Parasitol. 2014, 9, 194–201. [Google Scholar]
- Kot, K.; Łanocha-Arendarczyk, N.A.; Kosik-Bogacka, D.I. Amoebas from the genus Acanthamoeba and their pathogenic properties. Ann. Parasitol. 2018, 64, 299–308. [Google Scholar] [CrossRef]
- Balczun, C.; Scheid, P.L. Free-Living Amoebae as Hosts for and Vectors of Intracellular Microorganisms with Public Health Significance. Viruses 2017, 9, 65. [Google Scholar] [CrossRef]
- Scheid, P.; Balczun, C.; Schaub, G.A. Some secrets are revealed: Parasitic keratitis amoebae as vectors of the scarcely described pandoraviruses to humans. Parasitol. Res. 2014, 113, 3759–3764. [Google Scholar] [CrossRef]
- Rumelt, S.; Cohen, I.; Rehany, U. Spontaneous Corneal Graft Ulcerative Perforation Due to Mixed Acanthamoeba and Herpes Simplex Keratitis: A Clinicopathologic Study. Cornea 2000, 19, 240–242. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.; Peralta, J.M.; Peralta, R.H. Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, J.F.D. A Century of Research on the Amoeboflagellate Genus Naegleria. Acta Protozool. 2002, 41, 309. [Google Scholar]
- Dunn, A.L.; Reed, T.; Stewart, C.; Levy, R.A. Naegleria fowleriThat Induces Primary Amoebic Meningoencephalitis: Rapid Diagnosis and Rare Case of Survival in a 12-Year-Old Caucasian Girl. Lab. Med. 2016, 47, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liviac, D.; Wagner, E.D.; Mitch, W.A.; Altonji, M.J.; Plewa, M.J. Genotoxicity of Water Concentrates from Recreational Pools after Various Disinfection Methods. Environ. Sci. Technol. 2010, 44, 3527–3532. [Google Scholar] [CrossRef]
- CDC Healthy Swimming/Recreational Water. Available online: https://www.cdc.gov/healthywater/swimming/index.html (accessed on 8 March 2021).
- Ma, J.-W.; Huang, B.-S.; Hsu, C.-W.; Peng, C.-W.; Cheng, M.-L.; Kao, J.-Y.; Way, T.-D.; Yin, H.-C.; Wang, S.-S. Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. Int. J. Environ. Res. Public Health 2017, 14, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuy, M.; Berne, F.; Herbelin, P.; Binet, M.; Berthelot, N.; Rodier, M.-H.; Soreau, S.; Héchard, Y. Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int. J. Hyg. Environ. Health 2014, 217, 335–339. [Google Scholar] [CrossRef]
- Coulon, C.; Collignon, A.; McDonnell, G.; Thomas, V. Resistance of Acanthamoeba Cysts to Disinfection Treatments Used in Health Care Settings. J. Clin. Microbiol. 2010, 48, 2689–2697. [Google Scholar] [CrossRef] [Green Version]
- Greub, G.; Raoult, D. Microorganisms Resistant to Free-Living Amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef] [Green Version]
- Mogoa, E.; Bodet, C.; Morel, F.; Rodier, M.-H.; Legube, B.; Héchard, Y. Cellular Response of the Amoeba Acanthamoeba castellanii to Chlorine, Chlorine Dioxide, and Monochloramine Treatments. Appl. Environ. Microbiol. 2011, 77, 4974–4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agencia Estatal Boletín Oficial del Estado, Real Decreto 742/2013, de 27 de Septiembre, por el que se Establecen los Criterios técnico-Sanitarios de las Piscinas. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2013-10580 (accessed on 8 March 2021).
- Ahmad, T.; Shukla, K.; Sharma, A.K. Effect of Chlorine (Disinfectant) on Viability of Pathogenic Free Living Amoebae. Indian J. Fundam. Appl. Life Sci. 2012, 2, 132. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifaoui, I.; Rizo-Liendo, A.; Reyes-Batlle, M.; Arberas-Jiménez, I.; Rodríguez-Expósito, R.L.; Piñero, J.E.; Lorenzo-Morales, J. Effect of a Commercial Disinfectant CLORICAN® on Acanthamoeba spp. and Naegleria fowleri Viability. Parasitologia 2021, 1, 119-129. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030013
Sifaoui I, Rizo-Liendo A, Reyes-Batlle M, Arberas-Jiménez I, Rodríguez-Expósito RL, Piñero JE, Lorenzo-Morales J. Effect of a Commercial Disinfectant CLORICAN® on Acanthamoeba spp. and Naegleria fowleri Viability. Parasitologia. 2021; 1(3):119-129. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030013
Chicago/Turabian StyleSifaoui, Ines, Aitor Rizo-Liendo, María Reyes-Batlle, Iñigo Arberas-Jiménez, Rubén L. Rodríguez-Expósito, José E. Piñero, and Jacob Lorenzo-Morales. 2021. "Effect of a Commercial Disinfectant CLORICAN® on Acanthamoeba spp. and Naegleria fowleri Viability" Parasitologia 1, no. 3: 119-129. https://0-doi-org.brum.beds.ac.uk/10.3390/parasitologia1030013