Solvent-Mediated Structural Evolution Mechanism from Cs4PbBr6 to CsPbBr3 Crystals
Abstract
:1. Introduction
2. Experimental
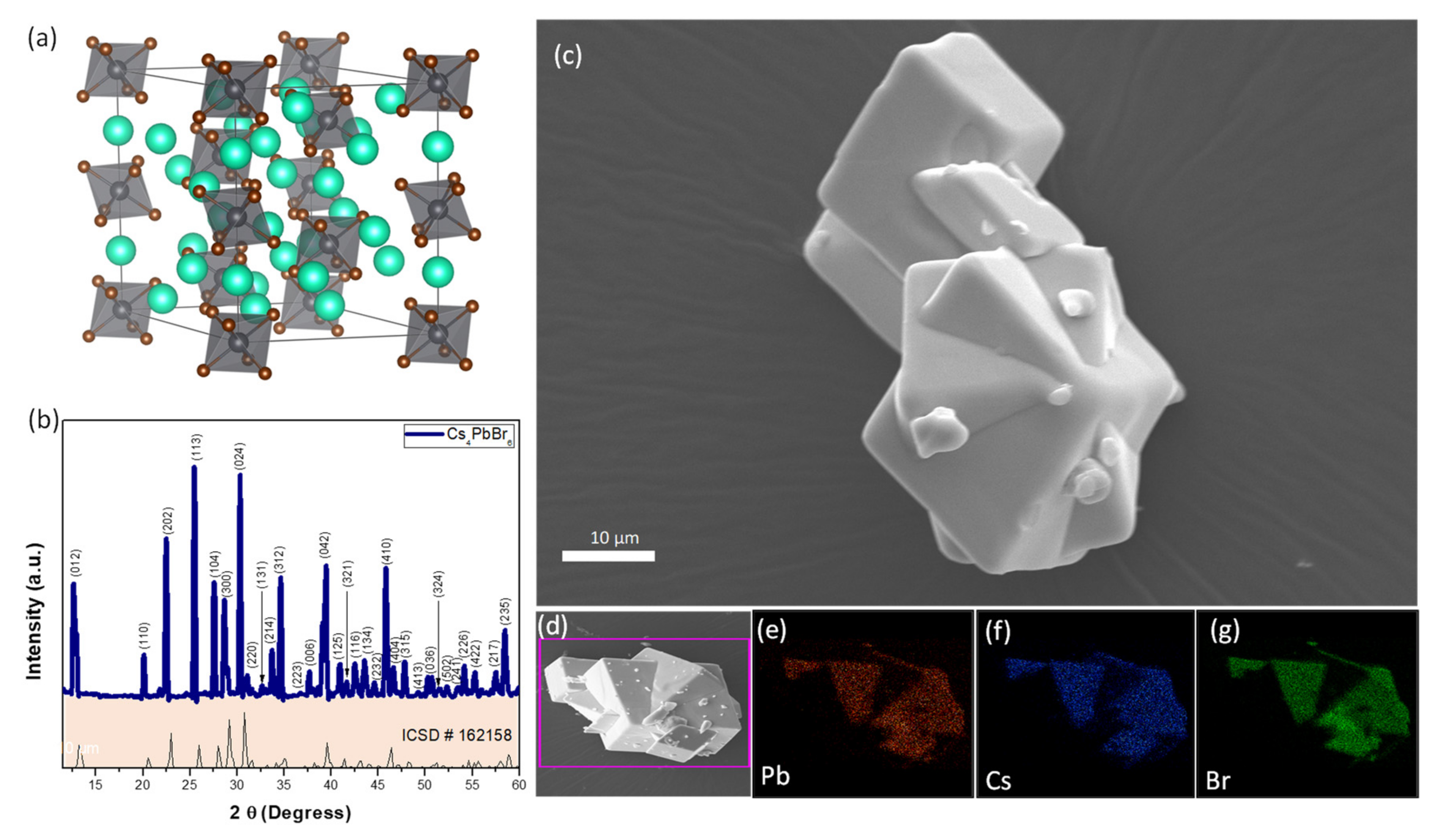
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; Gratzel, M. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 14, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Vale, B.R.C.; Socie, E.; Burgos-Caminal, A.; Bettini, J.; Schiavon, M.A.; Moser, J.-E. Exciton, Biexciton, and Hot Exciton Dynamics in CsPbBr3 Colloidal Nanoplatelets. J. Phys. Chem. Lett. 2020, 11, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Yu, D.; Xu, X.; Han, Z.; Zeng, H. CsPbBr3@Cs4PbBr6 Emitter-in-Host Composite: Fluorescence Origin and Interphase Energy Transfer. J. Phys. Chem. C 2021, 125, 3–19. [Google Scholar] [CrossRef]
- Yin, J.; Maity, P.; De Bastiani, M.; Dursum, I.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Molecular behavior of zero-dimensional perovskites. Sci. Adv. 2017, 3, e1701793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6(X = Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef]
- Cha, J.-H.; Han, J.H.; Yin, W.; Park, C.; Park, Y.; Ahn, T.K.; Cho, J.H.; Jung, D.-Y. Photoresponse of CsPbBr3 and Cs4PbBr6 Perovskite Single Crystals. J. Phys. Chem. Lett. 2017, 8, 565–570. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Wang, W.; Zheng, W.; Lin, R.; Huang, F. Growth, characterization and optoelectronic applications of pure-phase large-area CsPb2Br5 flake single crystals. J. Mater. Chem. C 2018, 6, 446–451. [Google Scholar] [CrossRef]
- Acharyya, P.; Pal, P.; Samanta, P.K.; Sarkar, A.; Pati, S.K.; Biswas, K. Single pot synthesis of indirect band gap 2D CsPb2Br5 nanosheets from direct band gap 3D CsPbBr3 nanocrystals and the origin of their luminescence properties. Nanoscale 2019, 11, 4001–4007. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr3 Solar Cells: Controlled Film Growth through Layer-by-Layer Quantum Dot Deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef] [Green Version]
- Gualdrón-Reyes, A.F.; Yoon, S.J.; Barea, E.M.; Agouram, S.; Muñoz-Sanjosé, V.; Meléndez, Á.M.; Niño-Gómez, M.E.; Mora-Seró, I. Controlling the Phase Segregation in Mixed Halide Perovskites through Nanocrystal Size. ACS Energy Lett. 2019, 4, 54–62. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Shi, Y.-L.; Li, M.; Zhang, L.; Wang, Z.-K. Vacuum-evaporated all-inorganic cesium lead bromine perovskites for high-performance light-emitting diodes. J. Mater. Chem. C 2017, 5, 8144–8149. [Google Scholar] [CrossRef]
- Sánchez, S.; Vallés-Pelarda, M.; Alberola-Borràs, J.-A.; Vidal, R.; Jerónimo-Rendón, J.J.; Saliba, M.; Boix, P.P.; Mora-Seró, I. Flash infrared annealing as a cost-effective and low environmental impact processing method for planar perovskite solar cells. Mater. Today 2019, 31, 39–46. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Bruno, A.; Soci, C.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Intrinsic Lead Ion Emissions in ZeroDimensional Cs4PbBr6 Nanocrystals. ACS Energy Lett. 2017, 2, 2805–2811. [Google Scholar] [CrossRef] [Green Version]
- Clasen, B.; Sánchez, R.; Fakharuddin, A.; Mora-Seró, I. A Comparative Study of Light-Emitting Diodes Based on All-Inorganic Perovskite Nanoparticles (CsPbBr3) Synthesized at Room Temperature and by a Hot-Injection Method. ChemPlusChem 2018, 83, 294–299. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, S.-G.; Lee, D.-K.; Park, N.-G. CH3NH3PbI3 and HC(NH2)2PbI3 Powders Synthesized from Low-Grade PbI2: Single Precursor for High-Efficiency Perovskite Solar Cells. ChemSusChem 2018, 11, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Alberola-Borràs, J.-A.; Mora-Seró, I. Abiotic depletion and the potential risk to the supply of cesium. Resour. Policy 2020, 68, 101792. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhang, Y.; Gao, H.; Yan, H. Large-area perovskite solar cells—A review of recent progress and issues. RSC Adv. 2018, 8, 10489–10508. [Google Scholar] [CrossRef] [Green Version]
- Demazeau, G. Solvothermal reactions: An original route for the synthesis of novel materials. J. Mater. Sci. 2008, 43, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Pinto, F.M.; Dey, S.; Duarte, T.M.; Taft, C.A.; La Porta, F.A. Perovskite-like quantum dots designed for advanced optoelectronic applications. In Functional Properties of Advanced Engineering Materials and Biomolecules; La Porta, F., Taft, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Dracopoulos, V.; Kastrissios, D.T.; Papatheodorou, G.N. Raman Spectra and Structure of PbCl2−ACl (A = K, Cs) Melts. Polyhedron 2005, 24, 619–625. [Google Scholar] [CrossRef]
- Velázquez, M.; Ferrier, A.; Péchev, S.; Gravereau, P.; Chaminade, J.-P.; Portier, X.; Moncorge, R. Growth and Characterization of Pure and Pr3+-Doped Cs4PbBr6 Crystals. J. Cryst. Growth 2008, 310, 5458–5463. [Google Scholar] [CrossRef]
- Jung, Y.-K.; Calbo, J.; Park, J.-S.; Whalley, L.D.; Kim, S.; Walsh, A. Intrinsic doping limit and defect-assisted luminescence in Cs4PbBr6. J. Mater. Chem. A 2019, 7, 20254–20261. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk-Aussig, F. Ein Beitrag zur Optik der Farban striche. Zeit. Tech. Phys. 1931, 12, 593. [Google Scholar]
- De Bastiani, M.; Dursun, I.; Zhang, Y.; Alshankiti, B.A.; Miao, X.-H.; Yin, J.; Yengel, E.; Alorousu, E.; Turedi, B.; Almutlaq, J.M.; et al. Inside Perovskites: Quantum Luminescence from Bulk Cs4PbBr6 Single Crystals. Chem. Mater. 2017, 29, 7108–7113. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.H.; Choi, S.H.; Park, W.K.; Yoo, J.S.; Kwon, S.B.; Kang, B.K.; Park, S.R.; Seo, Y.S.; Yang, W.S.; Yoon, D.H. Innovatively Continuous Mass Production Couette-taylor Flow: Pure Inorganic Green-Emitting Cs4PbBr6 Perovskite Microcrystal for display technology. Sci. Rep. 2018, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; La Porta, F.A. Recent Advances in Complex Functional Materials: From Design to Application; Springer: Cham, Switzerland, 2017; pp. 1–454. [Google Scholar]
- La Porta, F.A.; Taft, C.A. Emerging Research in Science and Engineering Based on Advanced Experimental and Computational Strategies; Springer: Cham, Switzerland, 2020; pp. 1–530. [Google Scholar]
- De Jesus, J.P.A.; Santos, A.C.L.; Pinto, F.M.; Taft, C.A.; La Porta, F.A. Review: Theoretical and experimental investigation of the intrinsic properties of Zn2GeO4 nanocrystals. J. Mater. Sci. 2021, 56, 4552–4568. [Google Scholar] [CrossRef]
- Mei, J.; Wang, F.; Wang, Y.; Tian, C.; Liu, H.; Zhao, D. Energy transfer assisted solvent effects on CsPbBr 3 quantum dots. J. Mater. Chem. C 2017, 5, 11076–11082. [Google Scholar] [CrossRef]
- Palazon, F.; Urso, C.; De Trizio, L.; Akkerman, Q.; Marras, S.; Locardi, F.; Nelli, I.; Ferretti, M.; Prato, M.; Manna, L. Postsynthesis Transformation of Insulating Cs4PbBr6 Nanocrystals into BrightPerovskite CsPbBr3 through Physical and Chemical Extraction of CsBr. ACS Energy Lett. 2017, 2, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Quintero-Bermudez, R.; Voznyy, O.; Walters, G.; Jain, A.; Fan, J.Z.; Zheng, X.; Yang, Z.; Sargent, E.H. Highly Emissive Green Perovskite Nanocrystals in a Solid State CrystallineMatrix. Adv. Mater. 2017, 29, 1605945. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Tan, L.; Wang, X.; Zhou, Y.; Xin, Y.; Ma, B.; Hanson, K.; Gao, H. Composite Perovskitesof Cesium Lead Bromide for Optimized Photoluminescence. J. Phys. Chem. Lett. 2017, 8, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.J.; Gösele, U.; Zacharias, M. Formation of nanotubes and hollow nanoparticles based on Kirkendall and diffusion processes: A review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O.; et al. A mechanistic study of phase transformation in perovskite nanocrystals driven by ligand passivation. Chem. Mater. 2018, 30, 84–93. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Porta, F.A.; Masi, S. Solvent-Mediated Structural Evolution Mechanism from Cs4PbBr6 to CsPbBr3 Crystals. Nanomanufacturing 2021, 1, 67-74. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1020007
La Porta FA, Masi S. Solvent-Mediated Structural Evolution Mechanism from Cs4PbBr6 to CsPbBr3 Crystals. Nanomanufacturing. 2021; 1(2):67-74. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1020007
Chicago/Turabian StyleLa Porta, Felipe A., and Sofia Masi. 2021. "Solvent-Mediated Structural Evolution Mechanism from Cs4PbBr6 to CsPbBr3 Crystals" Nanomanufacturing 1, no. 2: 67-74. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1020007





