Synthetic Approach to Rice Waste-Derived Carbon-Based Nanomaterials and Their Applications
Abstract
:1. Introduction
2. Carbon-Based Nanomaterials
3. Graphene
3.1. Synthesis of RH-Derived Graphene
3.1.1. Chemical Activation
3.1.2. Microwave-Assisted Method
3.1.3. Pyrolysis
3.2. Applications of RH-Derived Graphene
3.2.1. Desalination Membranes
3.2.2. Removal of Ni (II)
3.2.3. Gas Storage
3.2.4. Bactericidal Action
4. Carbon Nanotubes
4.1. Synthesis of RH-Derived Carbon Nanotubes
4.1.1. Chemical Vapor Deposition (CVD)
4.1.2. Microwave Oven Technique
4.1.3. Pyrolysis Technique
4.2. Potential Application of RH-Derived CNTs
4.2.1. Water Purification
Removal of Chemical Contaminants
Removal of Biological Contaminants/Microbial Decontamination
4.2.2. Supercapacitors
5. Carbon Dots
5.1. Synthesis of RH-Derived Carbon Dots (CDs, CQDs, GQDs)
5.1.1. Thermal Calcination
5.1.2. Microwave Hydrothermal
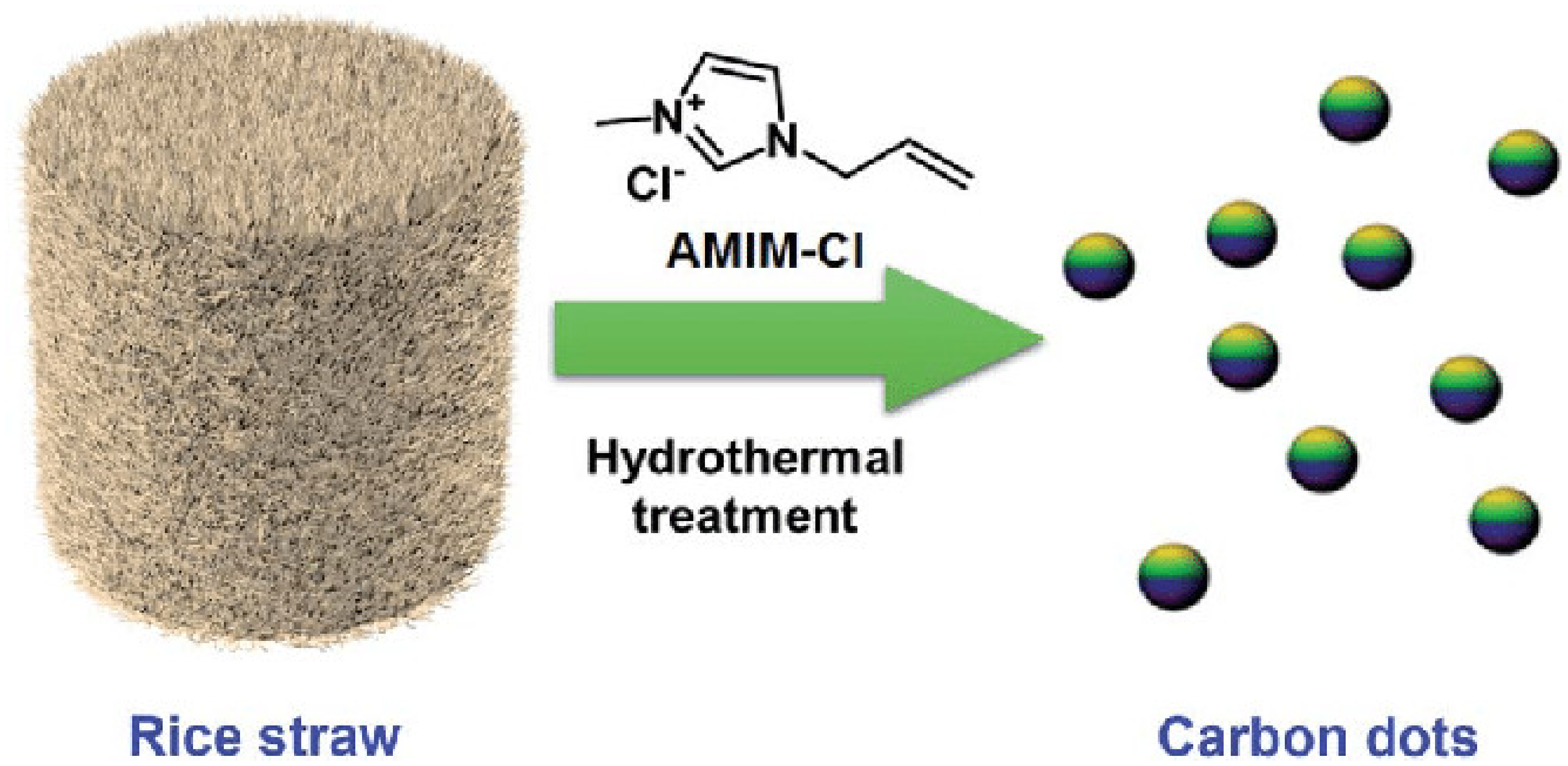
5.1.3. Hydrothermal Carbonization/Acid Oxidation
5.2. Applications of RH-Derived Carbon Dots
5.2.1. Bioimaging
5.2.2. Removal of Cadmium
5.2.3. Detection of Bacteria
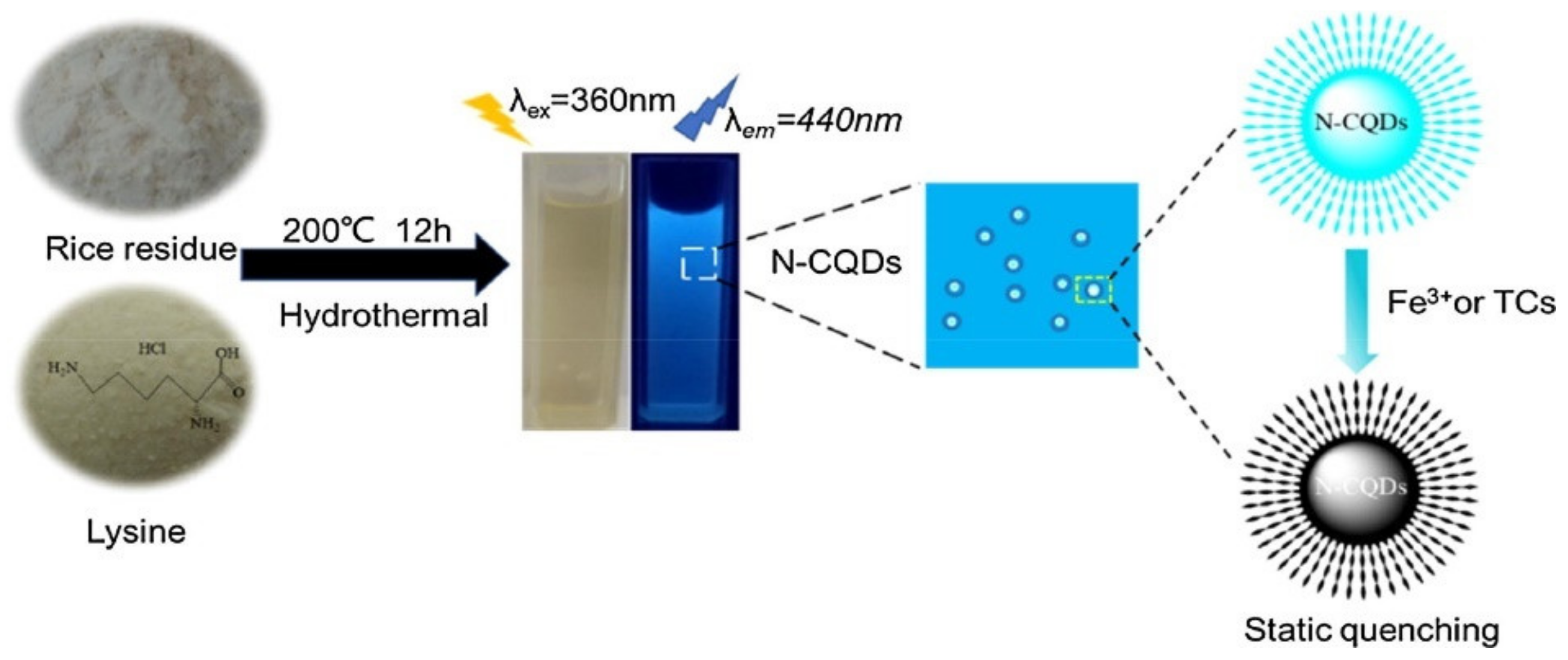
5.2.4. Fluorescent Probes for Setecting Fe3+ and Tetracycline
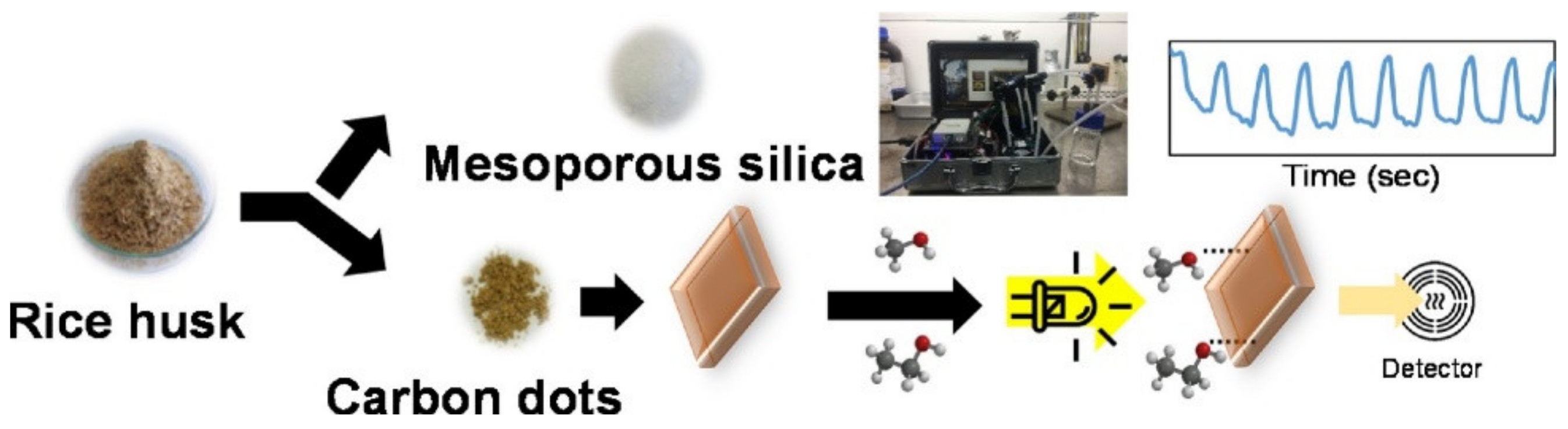
5.2.5. Detection of Volatile Organic Compounds (VOC) and Alcohol Vapors
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.; Chen, W. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review. Sep. Purif. Technol. 2020, 253, 117536. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Mattoso, L.H.; Moreira, F.K. Biomass-Derived Nanomaterials. In Nanostructured Materials for Energy Related Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 243–270. [Google Scholar]
- Wang, Y.; Sun, J.; He, B.; Feng, M. Synthesis and modification of biomass derived carbon dots in ionic liquids and their application: A mini review. Green Chem. Eng. 2020, 1, 94–108. [Google Scholar] [CrossRef]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photochem. Photobiol. B Biol. 2020, 209, 111940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Smith, A.T.; Wang, W.; Sun, L. Versatile nanostructures from rice husk biomass for energy applications. Angew. Chem. Int. Ed. 2018, 57, 13722–13734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Fathy, N.A.; Basta, A.H.; Lotfy, V.F. Novel trends for synthesis of carbon nanostructures from Agricultural wastes. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–74. [Google Scholar]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Zhou, J.; Yu, C.; Luo, Z.; Wang, Q.; Shi, Z. A novel two-staged thermal synthesis method of generating nanosilica from rice husk via pre-pyrolysis combined with calcination. Ind. Crop. Prod. 2015, 65, 1–6. [Google Scholar] [CrossRef]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Umeda, J.; Kondoh, K. High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal. Ind. Crop. Prod. 2010, 32, 539–544. [Google Scholar] [CrossRef]
- Sun, L.; Gong, K. Silicon-based materials from rice husks and their applications. Ind. Eng. Chem. Res. 2001, 40, 5861–5877. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M. Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: A comprehensive utilization of rice husk biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’Ondu, C.K.; Holt, C.M.; Olsen, B.C. Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kim, Y.A.; Yang, K.S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Terrones, M.; Endo, M.; Hayashi, T.; Saitoh, H. Rice husk-derived graphene with nano-sized domains and clean edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef]
- Rhee, I.; Kim, Y.A.; Shin, G.-O.; Kim, J.H.; Muramatsu, H. Compressive strength sensitivity of cement mortar using rice husk-derived graphene with a high specific surface area. Constr. Build. Mater. 2015, 96, 189–197. [Google Scholar] [CrossRef]
- Rhee, I.; Lee, J.-S.; Kim, J.H.; Kim, Y.A. Thermal performance, freeze-and-thaw resistance, and bond strength of cement mortar using rice husk-derived graphene. Constr. Build. Mater. 2017, 146, 350–359. [Google Scholar] [CrossRef]
- Hidalgo, P.; Navia, R.; Hunter, R.; Coronado, G.; Gonzalez, M. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J. Environ. Manag. 2019, 244, 83–91. [Google Scholar] [CrossRef]
- Debalina, B.; Reddy, R.B.; Vinu, R. Production of carbon nanostructures in biochar, bio-oil and gases from bagasse via microwave assisted pyrolysis using Fe and Co as susceptors. J. Anal. Appl. Pyrolysis 2017, 124, 310–318. [Google Scholar] [CrossRef]
- Wang, Z.; Ogata, H.; Morimoto, S.; Ortiz-Medina, J.; Fujishige, M.; Takeuchi, K.; Muramatsu, H.; Hayashi, T.; Terrones, M.; Hashimoto, Y. Nanocarbons from rice husk by microwave plasma irradiation: From graphene and carbon nanotubes to graphenated carbon nanotube hybrids. Carbon 2015, 94, 479–484. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.; Bourgeois, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2015, 17, 551–556. [Google Scholar] [CrossRef]
- Fathy, N.A. Carbon nanotubes synthesis using carbonization of pretreated rice straw through chemical vapor deposition of camphor. RSC Adv. 2017, 7, 28535–28541. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Yuan, M.; Zhong, R.; Gao, H.; Li, W.; Yun, X.; Liu, J.; Zhao, X.; Zhao, G.; Zhang, F. One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw, and their bio-applications in labeling, imaging, and sensing. Appl. Surf. Sci. 2015, 355, 1136–1144. [Google Scholar] [CrossRef]
- John, T.S.; Yadav, P.K.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence 2020, 35, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Kalita, H.; Mohapatra, J.; Pradhan, L.; Mitra, A.; Bahadur, D.; Aslam, M. Efficient synthesis of rice based graphene quantum dots and their fluorescent properties. RSC Adv. 2016, 6, 23518–23524. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Liu, J.; Peng, Y.; Yu, X.; Wang, W.; Zhang, Z.; Sun, L. One-pot facile synthesis of graphene quantum dots from rice husks for Fe3+ sensing. Ind. Eng. Chem. Res. 2018, 57, 9144–9150. [Google Scholar] [CrossRef]
- Mansurov, Z. Seitzhanova Makpal Azizovna; University of Naples Federico II: Naples, Italy, 2020. [Google Scholar]
- Seitzhanova, M.; Mansurov, Z.; Yeleuov, M.; Roviello, V.; Di Capua, R. The characteristics of graphene obtained from rice husk and graphite. Eurasian Chem.-Technol. J. 2019, 21, 149–156. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; Anis, B.; Youssef, M.A.; Abdallah, A.E.; El-Sakhawy, M.; Kamel, S. Preparation of eco-friendly graphene oxide from agricultural wastes for water treatment. Desalin. Water Treat. 2020, 191, 250–262. [Google Scholar] [CrossRef]
- Naik, M.; Debbarma, J.; Saha, M.; Bhargava, A. Graphene oxide nanoflakes from various agrowastes. Mater. Werkst. 2020, 51, 368–374. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9, S4. [Google Scholar] [CrossRef] [Green Version]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113. [Google Scholar] [CrossRef]
- Jiang, J.-W.; Leng, J.; Li, J.; Guo, Z.; Chang, T.; Guo, X.; Zhang, T. Twin graphene: A novel two-dimensional semiconducting carbon allotrope. Carbon 2017, 118, 370–375. [Google Scholar] [CrossRef]
- Siqueira, J.R.; Oliveira, O.N. 9—Carbon-Based Nanomaterials. In Nanostructures; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, UK, 2017; pp. 233–249. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G. Magical allotropes of carbon: Prospects and applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon nanotube assembly and integration for applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Huang, H.; Liu, Y.; Kang, Z. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E. Experimental review of graphene. Int. Sch. Res. Not. 2012, 2012, 501686. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, G. An introduction to the chemistry of graphene. Phys. Chem. Chem. Phys. 2015, 17, 28484–28504. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Kumar, S.; Sangwan, P.; Dhankhar, R.M.V.; Bidra, S. Utilization of rice husk and their ash: A review. Res. J. Chem. Environ. Sci 2013, 1, 126–129. [Google Scholar]
- Serra, M.F.; Conconi, M.S.; Gauna, M.R.; Suárez, G.; Aglietti, E.F.; Rendtorff, N. Mullite (3Al2O3·2SiO2) ceramics obtained by reaction sintering of rice husk ash and alumina, phase evolution, sintering and microstructure. J. Asian Ceram. Soc. 2016, 4, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Yusof, N.; Yusop, M.Z.M.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Karim, Z.A. Synthesis and characterization of graphene derived from rice husks. Malays. J. Fundam. Appl. Sci. 2019, 15, 516–521. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Arifin, N.F.T.; Yusof, N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Salleh, W.N.W. Graphene from waste and bioprecursors synthesis method and its application: A review. Malays. J. Fundam. Appl. Sci. 2020, 16, 342–350. [Google Scholar] [CrossRef]
- Raghavan, N.; Thangavel, S.; Venugopal, G. A short review on preparation of graphene from waste and bioprecursors. Appl. Mater. Today 2017, 7, 246–254. [Google Scholar] [CrossRef]
- Priyanka, M.; Saravanakumar, M. A short review on preparation and application of carbon foam. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032018. [Google Scholar] [CrossRef]
- Singh, P.; Bahadur, J.; Pal, K. One-step one chemical synthesis process of graphene from rice husk for energy storage applications. Graphene 2017, 6, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Uda, M.; Gopinath, S.C.; Hashim, U.; Uda, M.A.; hulwani Ibrahim, N.; Parmin, N.; Halim, N.; Anbu, P. Simple and Green Approach Strategy to Synthesis Graphene Using Rice Straw Ash. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012181. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Ismail, M.S.; Yusof, N.; Samitsu, S.; Yusop, M.Z.; Arifin, N.F.T.; Alias, N.H.; Jaafar, J.; Aziz, F.; Salleh, W.N.W. Methane adsorption by porous graphene derived from rice husk ashes under various stabilization temperatures. Carbon Lett. 2020, 30, 535–543. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process. Technol. 2020, 214, 106686. [Google Scholar] [CrossRef]
- Kumar, M.; Sachdeva, A.; Garg, R.K.; Singh, S. Synthesis and Characterization of Graphene Prepared from Rice Husk by a Simple Microwave Process. Nano Hybrids Compos. 2020, 29, 74–83. [Google Scholar] [CrossRef]
- Hashmi, A.; Singh, A.K.; Jain, B.; Singh, A. Muffle atmosphere promoted fabrication of graphene oxide nanoparticle by agricultural waste. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 627–636. [Google Scholar] [CrossRef]
- Zhou, H. Combustible Solid Waste Thermochemical Conversion: A Study of Interactions and Influence Factors; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. A Collect. Rev. Nat. J. 2010, 337–346. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- UN Water. The United Nations World Water Development Report 2014: Water and Energy; United Nations: Paris, France, 2014. [Google Scholar]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Xu, K.; Feng, B.; Zhou, C.; Huang, A. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination. Chem. Eng. Sci. 2016, 146, 159–165. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Nicolaï, A.; Sumpter, B.G.; Meunier, V. Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 2014, 16, 8646–8654. [Google Scholar] [CrossRef]
- Zheng, Z.; Grünker, R.; Feng, X. Synthetic two–dimensional materials: A new paradigm of membranes for ultimate separation. Adv. Mater. 2016, 28, 6529–6545. [Google Scholar] [CrossRef] [Green Version]
- Boretti, A.; Al-Zubaidy, S.; Vaclavikova, M.; Al-Abri, M.; Castelletto, S.; Mikhalovsky, S. Outlook for graphene-based desalination membranes. NPJ Clean Water 2018, 1, 5. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Thomas, M.; Corry, B.; Hilder, T.A. What have we learnt about the mechanisms of rapid water transport, ion rejection and selectivity in nanopores from molecular simulation? Small 2014, 10, 1453–1465. [Google Scholar] [CrossRef]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef]
- Wang, Q.; Kalantar-Zadeh, K.K.; Kis, A.A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Song, Z.; Zhou, F.; Yu, M. Polyamide/nitrogen-doped graphene oxide quantum dots (N-GOQD) thin film nanocomposite reverse osmosis membranes for high flux desalination. Desalination 2019, 451, 125–132. [Google Scholar] [CrossRef]
- McGuinness, N.B.; Garvey, M.; Whelan, A.; John, H.; Zhao, C.; Zhang, G.; Dionysiou, D.D.; Byrne, J.A.; Pillai, S.C. Nanotechnology solutions for global water challenges. In Water Challenges and Solutions on a Global Scale; ACS Publications: Washington, DC, USA, 2015; pp. 375–411. [Google Scholar]
- Raghav, S.; Painuli, R.; Kumar, D. Threats to Water: Issues and Challenges Related to Ground Water and Drinking Water. In A New Generation Material Graphene: Applications in Water Technology; Springer: Cham, Switzerland, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Al-Najar, B.; Bitra, R.B. Green and ecofriendly materials for the remediation of inorganic and organic pollutants in water. In A New Generation Material Graphene: Applications in Water Technology; Springer: Cham, Switzerland, 2019; pp. 69–110. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. Conventional to cutting edge technologies in Drinking Water Purification–a REVIEW. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 9375–9385. [Google Scholar]
- Aghigh, A.; Alizadeh, V.; Wong, H.Y.; Islam, M.S.; Amin, N.; Zaman, M. Recent advances in utilization of graphene for filtration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Baby Shaikh, R.; Saifullah, B.; Rehman, F.U. Greener method for the removal of toxic metal ions from the wastewater by application of agricultural waste as an adsorbent. Water 2018, 10, 1316. [Google Scholar] [CrossRef] [Green Version]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Chigo-Anota, E.; Salazar-Villanueva, M.; Hernández-Cocoletzi, H. Electronic Properties of Boron Nitride Oxide Nanoclusters. J. Nanosci. Nanotechnol. 2011, 11, 5515–5518. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramakrishnan, K.; Kirupha, S.D.; Sivanesan, S. Thermodynamic and kinetic studies of cadmium adsorption from aqueous solution onto rice husk. Braz. J. Chem. Eng. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.; Amin, M. Removal of heavy metals from wastewater using date palm as a biosorbent: A comparative review. Sains Malays. 2018, 47, 35–49. [Google Scholar]
- Lee, J.-Y.; Chen, C.-H.; Cheng, S.; Li, H.-Y. Adsorption of Pb (II) and Cu (II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2016, 13, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Economides, M.J.; Wood, D.A. The state of natural gas. J. Nat. Gas Sci. Eng. 2009, 1, 1–13. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S.; Abnisa, F.; Shafeeyan, M.S. Production of microporous palm shell based activated carbon for methane adsorption: Modeling and optimization using response surface methodology. Chem. Eng. Res. Des. 2012, 90, 776–784. [Google Scholar] [CrossRef]
- Policicchio, A.; Filosa, R.; Abate, S.; Desiderio, G.; Colavita, E. Activated carbon and metal organic framework as adsorbent for low-pressure methane storage applications: An overview. J. Porous Mater. 2017, 24, 905–922. [Google Scholar] [CrossRef]
- Choi, P.-S.; Jeong, J.-M.; Choi, Y.-K.; Kim, M.-S.; Shin, G.-J.; Park, S.-J. A review: Methane capture by nanoporous carbon materials for automobiles. Carbon Lett. 2016, 17, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, S.; Reyhani, A.; Mirershadi, S. Hydrogen storage properties of multi-walled carbon nanotubes and carbon nano-onions grown on single and bi-catalysts including Fe, Mo, Co and Ni supported by MgO. Int. J. Hydrog. Energy 2017, 42, 24885–24896. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, S. Yttrium dispersion on capped carbon nanotube: Promising materials for hydrogen storage applications. Int. J. Hydrog. Energy 2016, 41, 1053–1059. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Shiraz, M.G. Palladium nanoparticle and decorated carbon nanotube for electrochemical hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 11528–11533. [Google Scholar] [CrossRef]
- Hayashi, J.i.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Rios, R.B.; Silva, F.W.M.; Torres, A.E.B.; Azevedo, D.C.; Cavalcante, C.L. Adsorption of methane in activated carbons obtained from coconut shells using H 3 PO 4 chemical activation. Adsorption 2009, 15, 271–277. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Q.; Zhu, Z.; Hu, Y.; Tao, Z.; Chen, J. The enhanced hydrogen storage of micro-nanostructured hybrids of Mg (BH 4) 2–carbon nanotubes. Nanoscale 2015, 7, 18305–18311. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, Q. Methane adsorption on the graphene sheets, activated carbon and carbon black. Appl. Therm. Eng. 2016, 108, 605–613. [Google Scholar] [CrossRef]
- Durá, G.; Budarin, V.L.; Castro-Osma, J.A.; Shuttleworth, P.S.; Quek, S.C.; Clark, J.H.; North, M. Importance of Micropore–Mesopore Interfaces in Carbon Dioxide Capture by Carbon-Based Materials. Angew. Chem. 2016, 128, 9319–9323. [Google Scholar] [CrossRef]
- Che Othman, F.E.; Yusof, N.; Yub Harun, N.; Bilad, M.R.; Jaafar, J.; Aziz, F.; Wan Salleh, W.N.; Ismail, A.F. Novel activated carbon nanofibers composited with cost-effective graphene-based materials for enhanced adsorption performance toward methane. Polymers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Hosseinalipour, S.; Jabbari, E.; Madadelahi, M.; Fardad, A. Gas Mixing Simulation in a T-Shape Micro Channel Using The DSMC Method. Transp. Phenom. Nano Micro Scales 2014, 2, 132–139. [Google Scholar]
- Hillert, M.; Lange, N. The structure of graphite filaments. Z. Für Krist. Cryst. Mater. 1959, 111, 24–34. [Google Scholar] [CrossRef]
- Radushkevich, L.V.; Lukyanovich, V.M. The Structure of Carbon Forming in Thermal Decomposition of Carbon Monoxide on an Iron Catalyst. Russ. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Greil, P. Perspectives of nano-carbon based engineering materials. Adv. Eng. Mater. 2015, 17, 124–137. [Google Scholar] [CrossRef]
- Baughman, R.; Zakhidov, A.; Heer, W. Carbon Nanotubes-The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-W.; Aziz, A.; Chai, S.-P.; Mohamed, A.R.; Tye, C.-T. The effect of carbon precursors (methane, benzene and camphor) on the quality of carbon nanotubes synthesised by the chemical vapour decomposition. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1535. [Google Scholar] [CrossRef]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Hong, E.H.; Lee, K.-H.; Oh, S.H.; Park, C.-G. Synthesis of Carbon Nanotubes Using Microwave Radiation. Adv. Funct. Mater. 2003, 13, 961–966. [Google Scholar] [CrossRef]
- Omatola, K.; Onojah, A. Elemental analysis of rice husk ash using X-ray fluorescence technique. Int. J. Phys. Sci. 2009, 4, 189–193. [Google Scholar]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.H.; Lee, Y.H.; Kim, S.-G.; Rinzler, A.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [Green Version]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J.G. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 202. [Google Scholar] [CrossRef]
- Cho, W.S.; Hamada, E.; Kondo, Y.; Takayanagi, K. Synthesis of carbon nanotubes from bulk polymer. Appl. Phys. Lett. 1996, 69, 278–279. [Google Scholar] [CrossRef]
- Richter, H.; Hernádi, K.; Caudano, R.; Fonseca, A.; Migeon, H.; Nagy, J.; Schneider, S.; Vandooren, J.; Tiggelen, P.J. Formation of nanotubes in low pressure hydrocarbon flames. Carbon 1996, 34, 427–429. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Kosakovskaja, Z.J.; Fedorov, E.A.; Panov, V.I. New carbon tubelite-ordered film structure of multilayer nanotubes. Phys. Lett. A 1995, 197, 40–46. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M. (Ed.) Chapter 1—Deposition Technologies: An Overview. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 1–31. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Foul, A.A. A review: Production of activated carbon from agricultural byproducts via conventional and microwave heating. J. Chem. Technol. Biotechnol. 2013, 88, 1183–1190. [Google Scholar] [CrossRef]
- Asnawi, M.; Azhari, S.; Hamidon, M.N.; Ismail, I.; Helina, I. Synthesis of carbon nanomaterials from rice husk via microwave oven. J. Nanomater. 2018, 2018, 2898326. [Google Scholar] [CrossRef] [Green Version]
- Lotfy, V.F.; Fathy, N.A.; Basta, A.H. Novel approach for synthesizing different shapes of carbon nanotubes from rice straw residue. J. Environ. Chem. Eng. 2018, 6, 6263–6274. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Pan, B. Potential of carbon nanotubes in water treatment. Recent Prog. Carbon Nanotub. Res. 2012, 201110, 51332. [Google Scholar]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kaur, B.; Mehta, S.K. Potential prospects for carbon dots as a fluorescence sensing probe for metal ions. RSC Adv. 2016, 6, 90526–90536. [Google Scholar]
- Upadhyayula, V.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Vithanage, M.; Biswas, J.K.; Yi, H.; Dou, X.; Ok, Y.S. Sustainable sludge management by removing emerging contaminants from urban wastewater using carbon nanotubes. In Industrial and Municipal Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 553–571. [Google Scholar]
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z. European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci. Total Environ. 2017, 601, 1849–1868. [Google Scholar] [CrossRef]
- Sillanpää, M. Natural Organic Matter in Water: Characterization and Treatment Methods; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Leonard, P.; Hearty, S.; Brennan, J.; Dunne, L.; Quinn, J.; Chakraborty, T.; O’Kennedy, R. Advances in biosensors for detection of pathogens in food and water. Enzym. Microb. Technol. 2003, 32, 3–13. [Google Scholar] [CrossRef]
- De Paolis, F.; Kukkonen, J. Binding of organic pollutants to humic and fulvic acids: Influence of pH and the structure of humic material. Chemosphere 1997, 34, 1693–1704. [Google Scholar] [CrossRef]
- Dizge, N.; Tansel, B. Multiparametric investigation of competitive and noncompetitive sorption characteristics of SMP fractions (carbohydrate and protein) on activated carbon. J. Hazard. Mater. 2011, 185, 996–1004. [Google Scholar] [CrossRef]
- Cheng, W.; Dastgheib, S.A.; Karanfil, T. Adsorption of dissolved natural organic matter by modified activated carbons. Water Res. 2005, 39, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Dastgheib, S.A.; Karanfil, T.; Cheng, W. Tailoring activated carbons for enhanced removal of natural organic matter from natural waters. Carbon 2004, 42, 547–557. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Chen, H.; Yi, Z.; Xing, B. Sorption of humic acid to functionalized multi-walled carbon nanotubes. Environ. Pollut. 2013, 180, 1–6. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Loganathan, P.; Kazner, C.; Johir, M.; Vigneswaran, S. Submerged membrane filtration adsorption hybrid system for the removal of organic micropollutants from a water reclamation plant reverse osmosis concentrate. Desalination 2017, 401, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Shahid, S.; Shen, J.; Emanuelsson, E.A.C.; Patterson, D.A. A wide range and high resolution one-filtration molecular weight cut-off method for aqueous based nanofiltration and ultrafiltration membranes. J. Membr. Sci. 2017, 525, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.; Al-Wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef]
- Cho, H.-H.; Smith, B.A.; Wnuk, J.D.; Fairbrother, D.H.; Ball, W.P. Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 2899–2905. [Google Scholar] [CrossRef]
- Engel, M.; Chefetz, B. Adsorption and desorption of dissolved organic matter by carbon nanotubes: Effects of solution chemistry. Environ. Pollut. 2016, 213, 90–98. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Goodwin, D.; Marsh, K.; Fairbrother, D.H.; Schwab, K.J.; Jacangelo, J.G.; Huang, H. Modification of low pressure membranes with carbon nanotube layers for fouling control. Water Res. 2012, 46, 5645–5654. [Google Scholar] [CrossRef]
- Yang, X.; Lee, J.; Yuan, L.; Chae, S.-R.; Peterson, V.K.; Minett, A.I.; Yin, Y.; Harris, A.T. Removal of natural organic matter in water using functionalised carbon nanotube buckypaper. Carbon 2013, 59, 160–166. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wei, X. Influence of wastewater precoagulation on adsorptive filtration of pharmaceutical and personal care products by carbon nanotube membranes. Chem. Eng. J. 2018, 333, 66–75. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Liang, P.; Zhang, X.; Sun, J.; Wu, S.; Huang, X. In-situ combined dual-layer CNT/PVDF membrane for electrically-enhanced fouling resistance. J. Membr. Sci. 2015, 491, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Huang, H.; Cho, H.-H. Carbon nanotube composite membranes for microfiltration of pharmaceuticals and personal care products: Capabilities and potential mechanisms. J. Membr. Sci. 2015, 479, 165–174. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Liang, J.; Dai, J.; Liu, Z.; Xiong, W.; Wan, J.; Xu, P. Co-occurrence and interactions of pollutants, and their impacts on soil remediation—A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Lapworth, D.; Baran, N.; Stuart, M.; Ward, R. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Suárez, S.; Carballa, M.; Omil, F.; Lema, J.M. How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev. Environ. Sci. Bio/Technol. 2008, 7, 125–138. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Triantafillaki, S.; Dassenakis, M.; Androutsos, F.; Scoullos, M. Polycyclic aromatic hydrocarbons in surface seawater and in indigenous mussels (Mytilus galloprovincialis) from coastal areas of the Saronikos Gulf (Greece). Estuar. Coast. Shelf Sci. 2008, 79, 733–739. [Google Scholar] [CrossRef]
- Gimeno, R.; Marcé, R.; Borrull, F. Determination of organic contaminants in coastal water. TrAC Trends Anal. Chem. 2004, 23, 341–350. [Google Scholar] [CrossRef]
- Fromme, H.; Küchler, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Meyer, J.; Lacorte, S. Spatial distribution and sources of perfluorochemicals in the NW Mediterranean coastal waters (Catalonia, Spain). Environ. Pollut. 2010, 158, 2833–2840. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Stafiej, A.; Biesaga, M. Sorption behavior of acidic herbicides on carbon nanotubes. Microchim. Acta 2007, 159, 293–298. [Google Scholar] [CrossRef]
- Nam, S.-W.; Choi, D.-J.; Kim, S.-K.; Her, N.; Zoh, K.-D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Straathof, H.M. Investigations on the phytotoxic relevance of volatilization of herbicides. Meded. Fac. Landbouwwet. Rijksuniv. Gent 1986, 51, 433–438. [Google Scholar]
- Anju, A.; Ravi, S.P.; Bechan, S. Water pollution with special reference to pesticide contamination in India. J. Water Resour. Prot. 2010, 2010, 1793. [Google Scholar]
- Rocha, J.-D.R.; Rogers, R.E.; Dichiara, A.B.; Capasse, R.C. Emerging investigators series: Highly effective adsorption of organic aromatic molecules from aqueous environments by electronically sorted single-walled carbon nanotubes. Environ. Sci. Water Res. Technol. 2017, 3, 203–212. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J. Saudi Chem. Soc. 2017, 21, S179–S186. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, M.; Khajehsharifi, H.; Yadkuri, A.H.; Roosta, M.; Asghari, A. Oxidized multiwalled carbon nanotubes as efficient adsorbent for bromothymol blue. Toxicol. Environ. Chem. 2012, 94, 873–883. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. RSC Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef] [Green Version]
- Sadegh, H.; Zare, K.; Maazinejad, B.; Shahryari-Ghoshekandi, R.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT-COOH-Cysteamine composite and its application for dye removal. J. Mol. Liq. 2016, 215, 221–228. [Google Scholar] [CrossRef]
- Jahangiri-Rad, M.; Nadafi, K.; Mesdaghinia, A.; Nabizadeh, R.; Younesian, M.; Rafiee, M. Sequential study on reactive blue 29 dye removal from aqueous solution by peroxy acid and single wall carbon nanotubes: Experiment and theory. Iran. J. Environ. Health Sci. Eng. 2013, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.; Hilal, N.; Ismail, A. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef]
- Jame, S.A.; Zhou, Z. Electrochemical carbon nanotube filters for water and wastewater treatment. Nanotechnol. Rev. 2016, 5, 41–50. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, J.; Liu, F.; Huang, Y.; Li, G.; Zhang, J.; Chen, T. Robust preparation of superhydrophobic polymer/carbon nanotube hybrid membranes for highly effective removal of oils and separation of water-in-oil emulsions. J. Mater. Chem. A 2014, 2, 15268–15272. [Google Scholar] [CrossRef]
- Chen, X.; Hong, L.; Xu, Y.; Ong, Z.W. Ceramic pore channels with inducted carbon nanotubes for removing oil from water. ACS Appl. Mater. Interfaces 2012, 4, 1909–1918. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Rose, J.B. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 2004, 126, 219–234. [Google Scholar] [CrossRef]
- Richardson, S.D. Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends Anal. Chem. 2003, 22, 666–684. [Google Scholar] [CrossRef]
- Tian, C.; Liu, R.; Liu, H.; Qu, J. Disinfection by-products formation and precursors transformation during chlorination and chloramination of highly-polluted source water: Significance of ammonia. Water Res. 2013, 47, 5901–5910. [Google Scholar] [CrossRef]
- Hijnen, W.; Suylen, G.; Bahlman, J.; Brouwer-Hanzens, A.; Medema, G. GAC adsorption filters as barriers for viruses, bacteria and protozoan (oo) cysts in water treatment. Water Res. 2010, 44, 1224–1234. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Karanfil, T. The effects of dissolved natural organic matter on the adsorption of synthetic organic chemicals by activated carbons and carbon nanotubes. Water Res. 2011, 45, 1378–1386. [Google Scholar] [CrossRef]
- Osman, A.I.; Farrell, C.; Ala’a, H.; Harrison, J.; Rooney, D.W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020, 10, 2563. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Optimization of parameters for adsorption of metal ions onto rice husk ash using Taguchi’s experimental design methodology. Chem. Eng. J. 2008, 140, 136–144. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.-h.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Lu, C.; Su, F. Adsorption of natural organic matter by carbon nanotubes. Sep. Purif. Technol. 2007, 58, 113–121. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Luo, T.; Zhang, Y.-X.; Jia, Y.; Zhu, B.-J.; Fu, X.-C.; Liu, J.-H.; Huang, X.-J. Adsorption of lead (II) on O2-plasma-oxidized multiwalled carbon nanotubes: Thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces 2011, 3, 2585–2593. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.; Sankararamakrishnan, N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard. Mater. 2014, 274, 132–144. [Google Scholar] [CrossRef]
- Pillay, K.; Cukrowska, E.; Coville, N. Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2009, 166, 1067–1075. [Google Scholar] [CrossRef]
- Farid, M.U.; Luan, H.-Y.; Wang, Y.; Huang, H.; An, A.K.; Khan, R.J. Increased adsorption of aqueous zinc species by Ar/O2 plasma-treated carbon nanotubes immobilized in hollow-fiber ultrafiltration membrane. Chem. Eng. J. 2017, 325, 239–248. [Google Scholar] [CrossRef]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr (VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalination Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Al Amer, A.M.; Laoui, T.; Abbas, A.; Al-Aqeeli, N.; Patel, F.; Khraisheh, M.; Atieh, M.A.; Hilal, N. Fabrication and antifouling behaviour of a carbon nanotube membrane. Mater. Des. 2016, 89, 549–558. [Google Scholar]
- Bahgat, M.; Farghali, A.; El Rouby, W.; Khedr, M. Synthesis and modification of multi-walled carbon nano-tubes (MWCNTs) for water treatment applications. J. Anal. Appl. Pyrolysis 2011, 92, 307–313. [Google Scholar] [CrossRef]
- Gupta, V.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Adsorption of zinc (II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb (II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Mitra, S. Removal of trace arsenic to meet drinking water standards using iron oxide coated multiwall carbon nanotubes. J. Chem. Eng. Data 2011, 56, 2077–2083. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef]
- Deng, S.; Upadhyayula, V.K.; Smith, G.B.; Mitchell, M.C. Adsorption equilibrium and kinetics of microorganisms on single-wall carbon nanotubes. IEEE Sens. J. 2008, 8, 954–962. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; You, H.; Zhao, X. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environ. Toxicol. Pharmacol. 2013, 36, 451–462. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Liu, T.; Tang, H.; Cai, X.; Zhao, J.; Li, D.; Li, R.; Sun, X. A study on bactericidal properties of Ag coated carbon nanotubes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 264, 282–286. [Google Scholar] [CrossRef]
- Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.; Saleh, T.A.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Adsorptive removal of cadmium (II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 255–263. [Google Scholar]
- Laoui, T.; Al-Amer, A.M.; Khalil, A.B.; Abbas, A.; Khraisheh, M.; Atieh, M.A. Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane. Desalination 2015, 376, 82–93. [Google Scholar]
- Ahmed, F.; Santos, C.M.; Mangadlao, J.; Advincula, R.; Rodrigues, D.F. Antimicrobial PVK: SWNT nanocomposite coated membrane for water purification: Performance and toxicity testing. Water Res. 2013, 47, 3966–3975. [Google Scholar] [CrossRef]
- Aslan, S.; Deneufchatel, M.; Hashmi, S.; Li, N.; Pfefferle, L.D.; Elimelech, M.; Pauthe, E.; Van Tassel, P.R. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J. Colloid Interface Sci. 2012, 388, 268–273. [Google Scholar] [CrossRef]
- Mostafavi, S.; Mehrnia, M.; Rashidi, A. Preparation of nanofilter from carbon nanotubes for application in virus removal from water. Desalination 2009, 238, 271–280. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Nguyen, T.H.; Gutierrez, L.; Elimelech, M. Impact of solution chemistry on viral removal by a single-walled carbon nanotube filter. Water Res. 2010, 44, 3773–3780. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Alvarez, P.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. MnO2/g-C3N4 nanocomposite with highly enhanced supercapacitor performance. Nanotechnology 2017, 28, 135705. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Y.; Zheng, S.; Guo, X.; Xue, H.; Pang, H. Recent progress in some amorphous materials for supercapacitors. Small 2018, 14, 1800426. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, K.; Bavani, T.; Arunachalam, P.; Lee, S.J.; Theerthagiri, J.; Madhavan, J.; Pollet, B.G.; Choi, M.Y. Nanofiber NiMoO4/g-C3N4 composite electrode materials for redox supercapacitor applications. Nanomaterials 2020, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Banerjee, S.; De, B.; Sinha, P.; Cherusseri, J.; Kar, K.K. Applications of Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials I; Springer: Berlin/Heidelberg, Germany, 2020; pp. 341–350. [Google Scholar]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Guan, L.; Yu, L.; Chen, G.Z. Capacitive and non-capacitive faradaic charge storage. Electrochim. Acta 2016, 206, 464–478. [Google Scholar] [CrossRef]
- You, B.; Wang, L.; Yao, L.; Yang, J. Three dimensional N-doped graphene–CNT networks for supercapacitor. Chem. Commun. 2013, 49, 5016–5018. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Kim, B.N.; Kim, I.G.; You, I.-K.; Kim, T. Activated biomass-derived graphene-based carbons for supercapacitors with high energy and power density. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Barisci, J.N.; Wallace, G.G.; Baughman, R.H. Electrochemical characterization of single-walled carbon nanotube electrodes. J. Electrochem. Soc. 2000, 147, 4580. [Google Scholar] [CrossRef]
- Yu, D.; Qian, Q.; Wei, L.; Jiang, W.; Goh, K.; Wei, J.; Zhang, J.; Chen, Y. Emergence of fiber supercapacitors. Chem. Soc. Rev. 2015, 44, 647–662. [Google Scholar] [CrossRef]
- Niu, C.; Sichel, E.K.; Hoch, R.; Moy, D.; Tennent, H. High power electrochemical capacitors based on carbon nanotube electrodes. Appl. Phys. Lett. 1997, 70, 1480–1482. [Google Scholar] [CrossRef]
- Cherusseri, J.; Sharma, R.; Kar, K.K. Nanotechnology advancements on carbon nanotube/polypyrrole composite electrodes for supercapacitors. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 479–510. [Google Scholar]
- Pan, H.; Li, J.; Feng, Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K.; Kim, W. Recent progress and futuristic development of PbSe thermoelectric materials and devices. Mater. Today Energy 2018, 9, 359–376. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Sohn, J.I.; Kim, Y.-S.; Shim, H.-S.; Kim, W.B.; Seong, T.-Y. Electrochemical capacitors fabricated with carbon nanotubes grown within the pores of anodized aluminum oxide templates. Electrochem. Commun. 2006, 8, 513–516. [Google Scholar] [CrossRef]
- Jung, M.; Kim, H.-G.; Lee, J.-K.; Joo, O.-S.; Mho, S.-i. EDLC characteristics of CNTs grown on nanoporous alumina templates. Electrochim. Acta 2004, 50, 857–862. [Google Scholar] [CrossRef]
- Banerjee, S.; Sharma, R.; Kar, K.K. Nanocomposites based on carbon nanomaterials and electronically nonconducting polymers. In Composite Materials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 251–280. [Google Scholar]
- Li, C.; Wang, D.; Liang, T.; Wang, X.; Wu, J.; Hu, X.; Liang, J. Oxidation of multiwalled carbon nanotubes by air: Benefits for electric double layer capacitors. Powder Technol. 2004, 142, 175–179. [Google Scholar] [CrossRef]
- Hola, K.; Bourlinos, A.B.; Kozak, O.; Berka, K.; Siskova, K.M.; Havrdova, M.; Tucek, J.; Safarova, K.; Otyepka, M.; Giannelis, E.P. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO− induced red-shift emission. Carbon 2014, 70, 279–286. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft–hard template approach. Chem. Commun. 2013, 49, 4920–4922. [Google Scholar] [CrossRef]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef]
- Duran, N.; Simoes, M.B.; de Moraes, A.; Favaro, W.J.; Seabra, A.B. Nanobiotechnology of carbon dots: A review. J. Biomed. Nanotechnol. 2016, 12, 1323–1347. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Wu, C.; Gu, S. State-of-the-Art on the Preparation, Modification, and Application of Biomass-Derived Carbon Quantum Dots. Ind. Eng. Chem. Res. 2020, 59, 22017–22039. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Zhang, J.; Li, Z.; Chen, J.; Liu, P.; Wu, D. An ionic liquid promoted microwave-hydrothermal route towards highly photoluminescent carbon dots for sensitive and selective detection of iron (III). RSC Adv. 2015, 5, 24205–24209. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Guerreiro, M.; Duarte, F.J.; Freire, C.S.; Calhorda, M.J.; Silvestre, A.J.; Kunz, W.; Rebelo, L.P.N. Unveiling the dual role of the cholinium hexanoate ionic liquid as solvent and catalyst in suberin depolymerisation. RSC Adv. 2014, 4, 2993–3002. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N. Rapid detection of bacteria by carbon quantum dots. J. Biomed. Nanotechnol. 2011, 7, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Choudhury, M.; Nath, S.; Chakdar, D.; Gope, G.; Nath, R. Acetone sensing of ZnO quantum dots embedded in polyvinyl alcohol matrix. Adv. Sci. Lett. 2010, 3, 6–9. [Google Scholar] [CrossRef]
- Thongsai, N.; Tanawannapong, N.; Praneerad, J.; Kladsomboon, S.; Jaiyong, P.; Paoprasert, P. Real-time detection of alcohol vapors and volatile organic compounds via optical electronic nose using carbon dots prepared from rice husk and density functional theory calculation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 278–287. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk–natural proteins. J. Mater. Chem. B 2013, 1, 2868–2873. [Google Scholar] [CrossRef]
- Pal, T.; Mohiyuddin, S.; Packirisamy, G. Facile and green synthesis of multicolor fluorescence carbon dots from curcumin: In vitro and in vivo bioimaging and other applications. ACS Omega 2018, 3, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Abidin, N.H.Z.; Wongso, V.; Hui, K.C.; Cho, K.; Sambudi, N.S.; Ang, W.L.; Saad, B. The effect of functionalization on rice-husks derived carbon quantum dots properties and cadmium removal. J. Water Process Eng. 2020, 38, 101634. [Google Scholar] [CrossRef]
- Naik, J.P.; Sutradhar, P.; Saha, M. Molecular scale rapid synthesis of graphene quantum dots (GQDs). J. Nanostructure Chem. 2017, 7, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, J.; Wang, W.; Wei, Z.; Wang, F.; Gong, P.; Wang, J.; Li, N.; Liu, B.; Zhang, Z. Photoluminescent carbon quantum dot grafted silica nanoparticles directly synthesized from rice husk biomass. J. Mater. Chem. B 2017, 5, 4679–4689. [Google Scholar] [CrossRef]
- Wongso, V.; Sambudi, N.; Sufian, S.; Abdullah, B. The effect of pH in the synthesis of carbon quantum dots from rice husk on their photoluminescence properties. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012087. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Li, K.; Zhao, X.; Wei, G.; Su, Z. Recent advances in the cancer bioimaging with graphene quantum dots. Curr. Med. Chem. 2018, 25, 2876–2893. [Google Scholar] [CrossRef]
- Feng, L.-L.; Wu, Y.-X.; Zhang, D.-L.; Hu, X.-X.; Zhang, J.; Wang, P.; Song, Z.-L.; Zhang, X.-B.; Tan, W. Near infrared graphene quantum dots-based two-photon nanoprobe for direct bioimaging of endogenous ascorbic acid in living cells. Anal. Chem. 2017, 89, 4077–4084. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, Y.; Routh, P.; Sk, M.A.; Than, A.; Lin, M.; Zhang, J.; Chen, J.; Sun, H.; Chen, P. Nitrogen and phosphorus co-doped graphene quantum dots: Synthesis from adenosine triphosphate, optical properties, and cellular imaging. Nanoscale 2015, 7, 8159–8165. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Periasamy, A.P.; Chuang, C.; Liou, Y.-R.; Chen, Y.-F.; Joly, J.; Liang, C.-T.; Chang, H.-T. Plant leaf-derived graphene quantum dots and applications for white LEDs. New J. Chem. 2014, 38, 4946–4951. [Google Scholar] [CrossRef]
- Rahbari, R.; Sheahan, T.; Modes, V.; Collier, P.; Macfarlane, C.; Badge, R.M. A novel L1 retrotransposon marker for HeLa cell line identification. Biotechniques 2009, 46, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ngu, P.Z.Z.; Chia, S.P.P.; Fong, J.F.Y.; Ng, S.M. Synthesis of carbon nanoparticles from waste rice husk used for the optical sensing of metal ions. New Carbon Mater. 2016, 31, 135–143. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, M.; Zhu, T.; Tang, X.; Han, S.; Huang, W.; Shi, Y.; Liu, A. The synthesis of water-dispersible zinc doped AgInS2 quantum dots and their application in Cu2+ detection. J. Lumin. 2017, 192, 547–554. [Google Scholar] [CrossRef]
- Kahrizi, P.; Mohseni-Shahri, F.S.; Moeinpour, F. Adsorptive removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. J. Nanostructure Chem. 2018, 8, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Long, P.; Feng, Y.; Qin, C.; Feng, W. Surface passivation of carbon dots with ethylene glycol and their high-sensitivity to Fe3+. RSC Adv. 2017, 7, 2810–2816. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, H.; Teleb, N.; Yahia, I.; Zahran, H.; Elhaes, H.; Ibrahim, M. First principles study of the adsorption of hydrated heavy metals on graphene quantum dots. J. Phys. Chem. Solids 2019, 130, 32–40. [Google Scholar] [CrossRef]
- Qiao, X.; Huang, W.; Bian, Y. Effective removal of cadmium ions from a simulated gastrointestinal fluid by Lentinus edodes. Int. J. Environ. Res. Public Health 2014, 11, 12486–12498. [Google Scholar] [CrossRef] [Green Version]
- Shtepliuk, I.; Caffrey, N.M.; Iakimov, T.; Khranovskyy, V.; Abrikosov, I.A.; Yakimova, R. On the interaction of toxic Heavy Metals (Cd, Hg, Pb) with graphene quantum dots and infinite graphene. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Xia, Y. Facile synthesis of nitrogen-doped carbon quantum dots with chitosan for fluorescent detection of Fe3+. Polymers 2019, 11, 1731. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Li, Y.-K.; Chen, M.-L.; Wang, J.-H. Supported carbon dots decorated with metallothionein for selective cadmium adsorption and removal. Chin. Chem. Lett. 2015, 26, 1496–1501. [Google Scholar] [CrossRef]
- Liu, B.R.; Li, J.-F.; Lu, S.-W.; Lee, H.-J.; Huang, Y.-W.; Shannon, K.B.; Aronstam, R.S. Cellular internalization of quantum dots noncovalently conjugated with arginine-rich cell-penetrating peptides. J. Nanosci. Nanotechnol. 2010, 10, 6534–6543. [Google Scholar] [CrossRef] [Green Version]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators. Nano Lett. 2007, 7, 1344–1348. [Google Scholar] [CrossRef]
- Liu, H.Y.; Vu, T.Q. Identification of quantum dot bioconjugates and cellular protein co-localization by hybrid gel blotting. Nano Lett. 2007, 7, 1044–1049. [Google Scholar] [CrossRef]
- Gratieri, T.; Schaefer, U.F.; Jing, L.; Gao, M.; Kostka, K.-H.; Lopez, R.F.; Schneider, M. Penetration of quantum dot particles through human skin. J. Biomed. Nanotechnol. 2010, 6, 586–595. [Google Scholar] [CrossRef]
- Mortensen, L.J.; Ravichandran, S.; Zheng, H.; DeLouise, L.A. Progress and challenges in quantifying skin permeability to nanoparticles using a quantum dot model. J. Biomed. Nanotechnol. 2010, 6, 596–604. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, G.; DeLouise, L.A.; Lou, Z. Detection of the cancer marker CD146 expression in melanoma cells with semiconductor quantum dot label. J. Biomed. Nanotechnol. 2010, 6, 303–311. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157–4162. [Google Scholar] [CrossRef]
- Feng, Y.; Zhong, D.; Miao, H.; Yang, X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta 2015, 140, 128–133. [Google Scholar] [CrossRef]
- Canhoto, O.; Pinzari, F.; Fanelli, C.; Magan, N. Application of electronic nose technology for the detection of fungal contamination in library paper. Int. Biodeterior. Biodegrad. 2004, 54, 303–309. [Google Scholar] [CrossRef]
- Thaler, E.R.; Kennedy, D.W.; Hanson, C.W. Medical applications of electronic nose technology: Review of current status. Am. J. Rhinol. 2001, 15, 291–295. [Google Scholar] [CrossRef]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. LWT-Food Sci. Technol. 1998, 31, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]

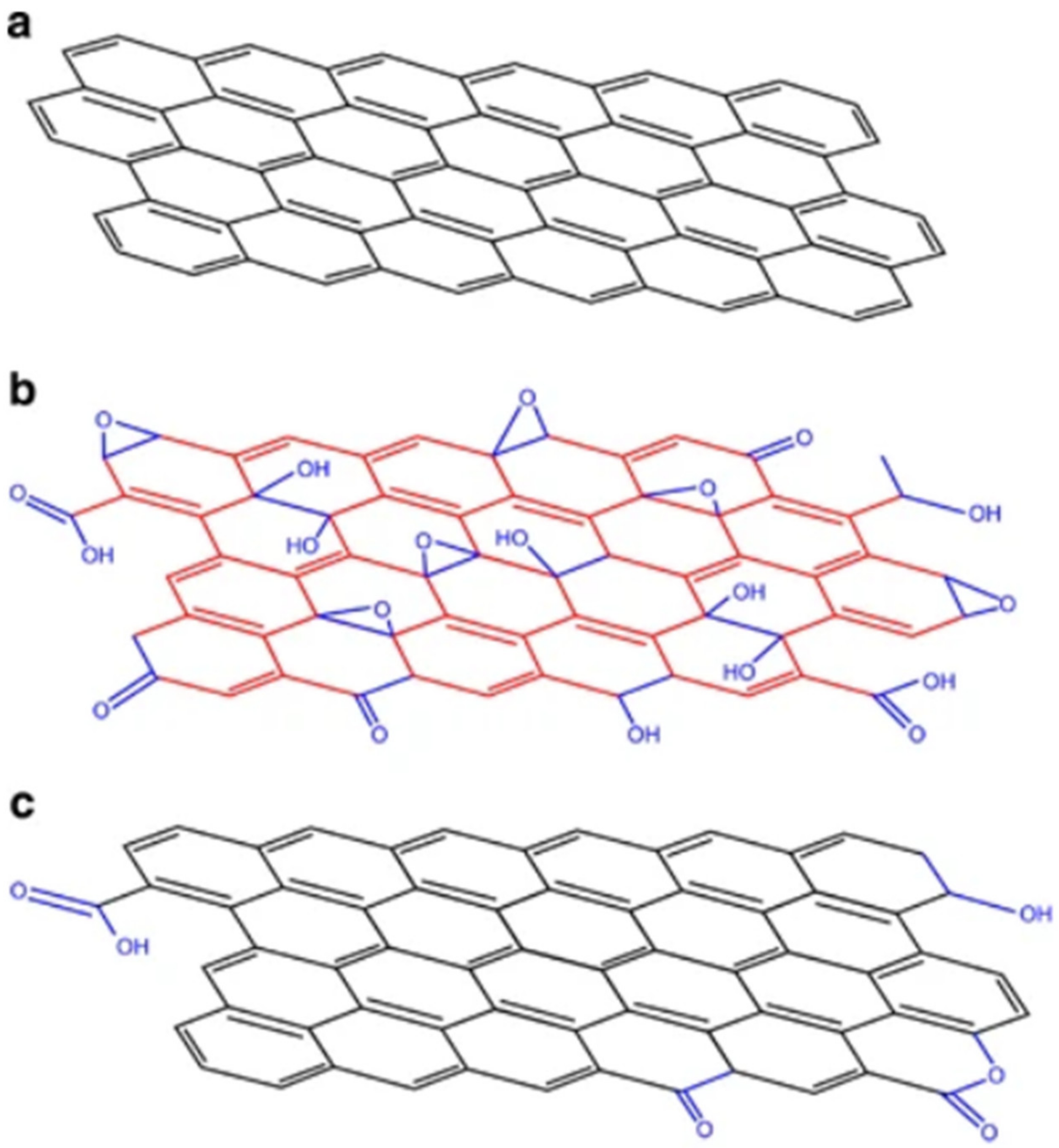
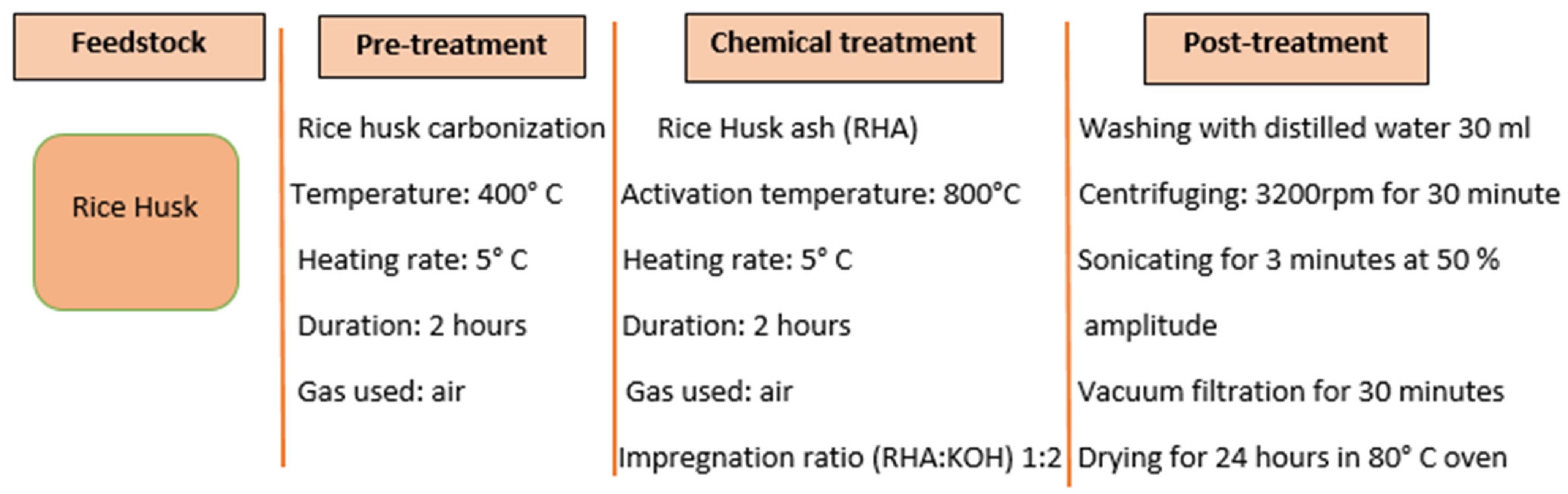
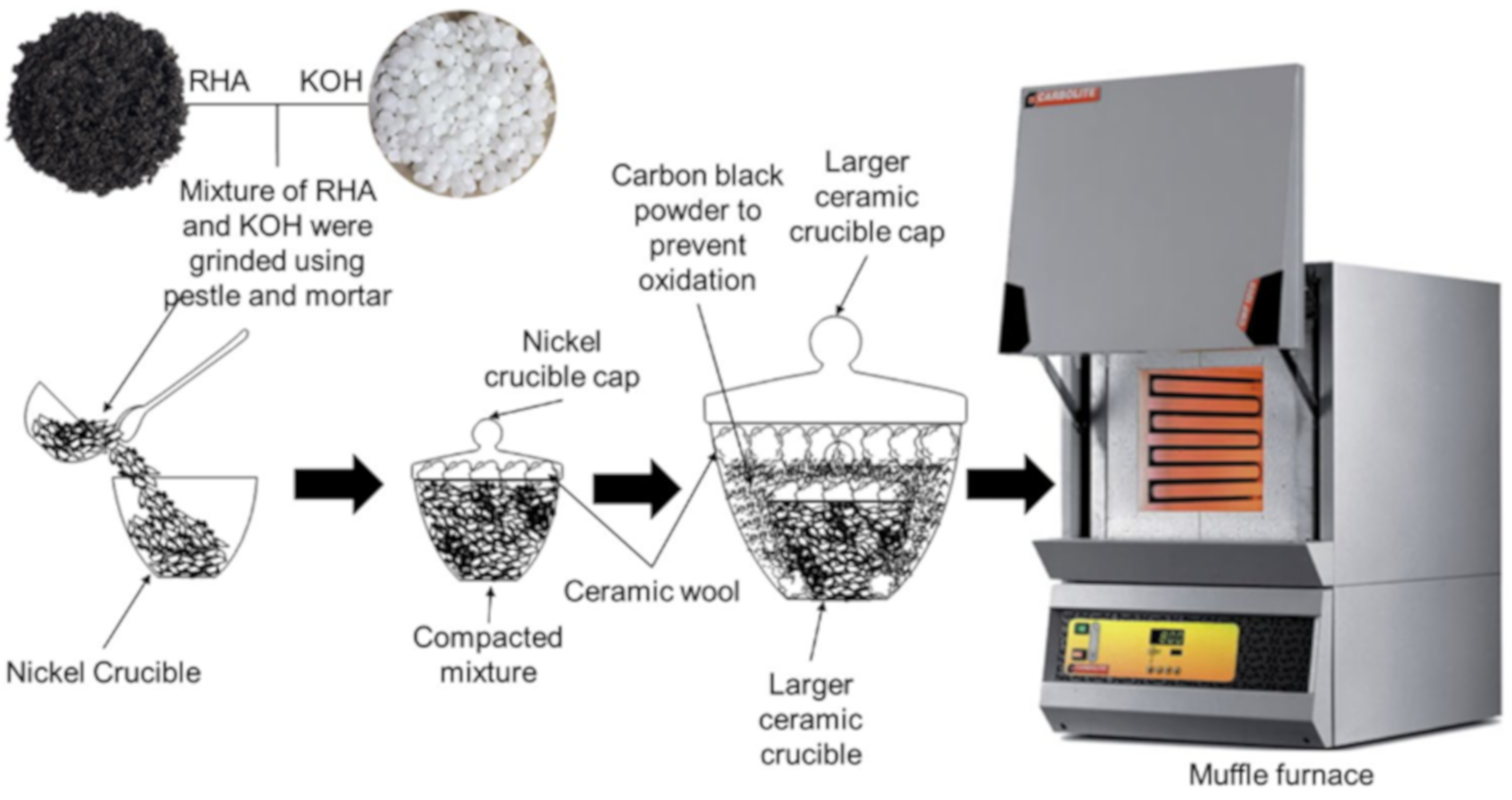


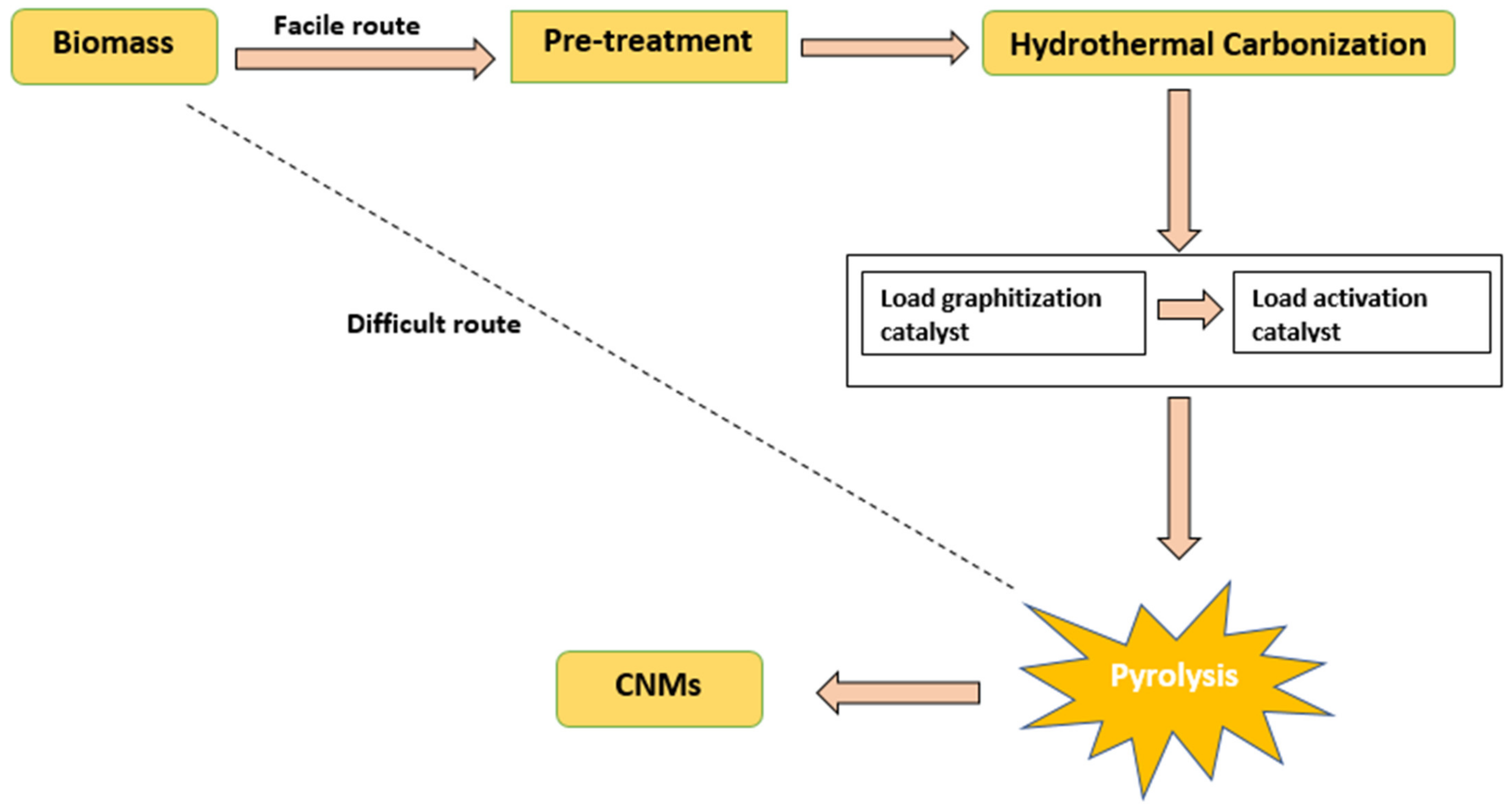


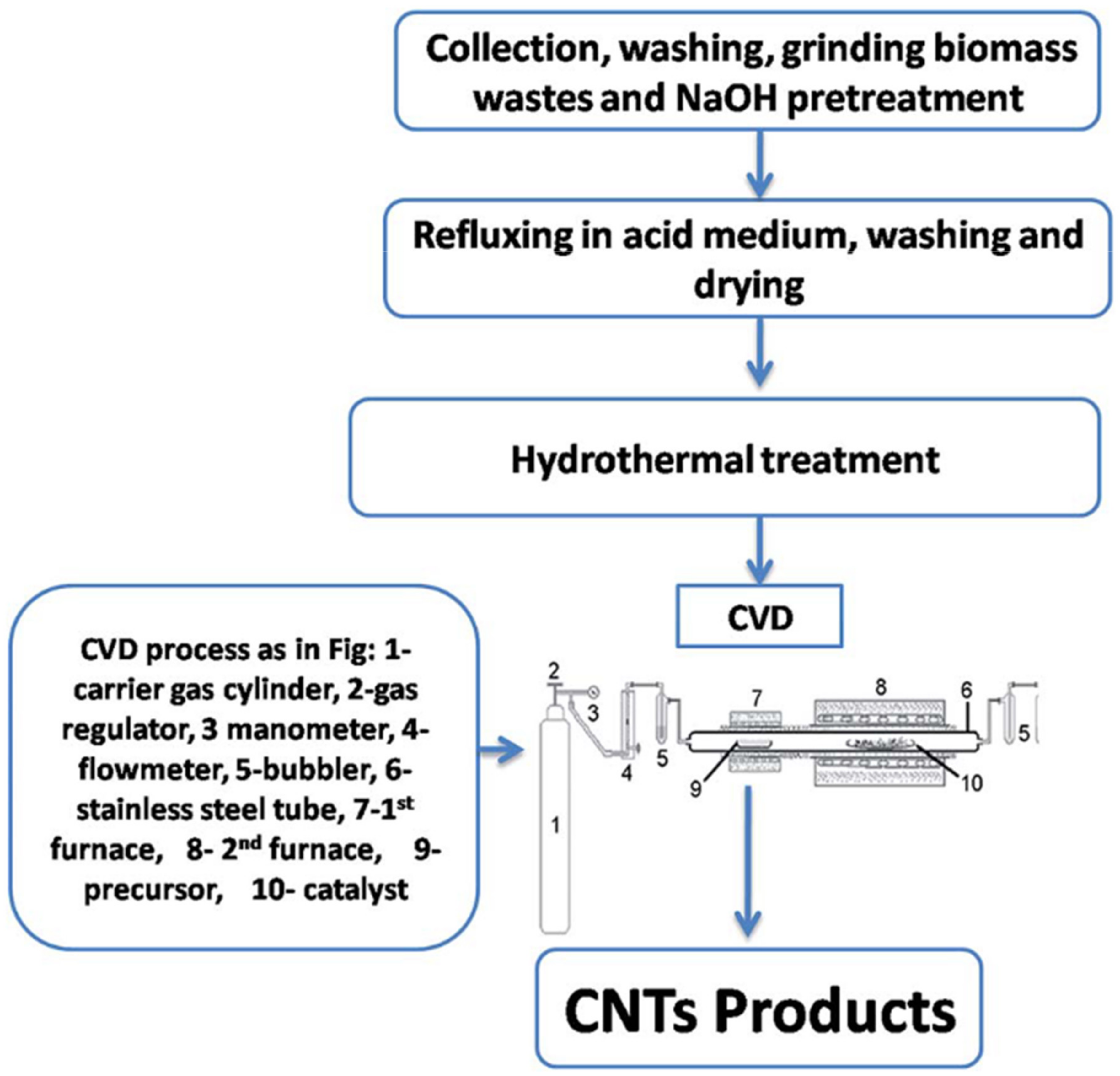
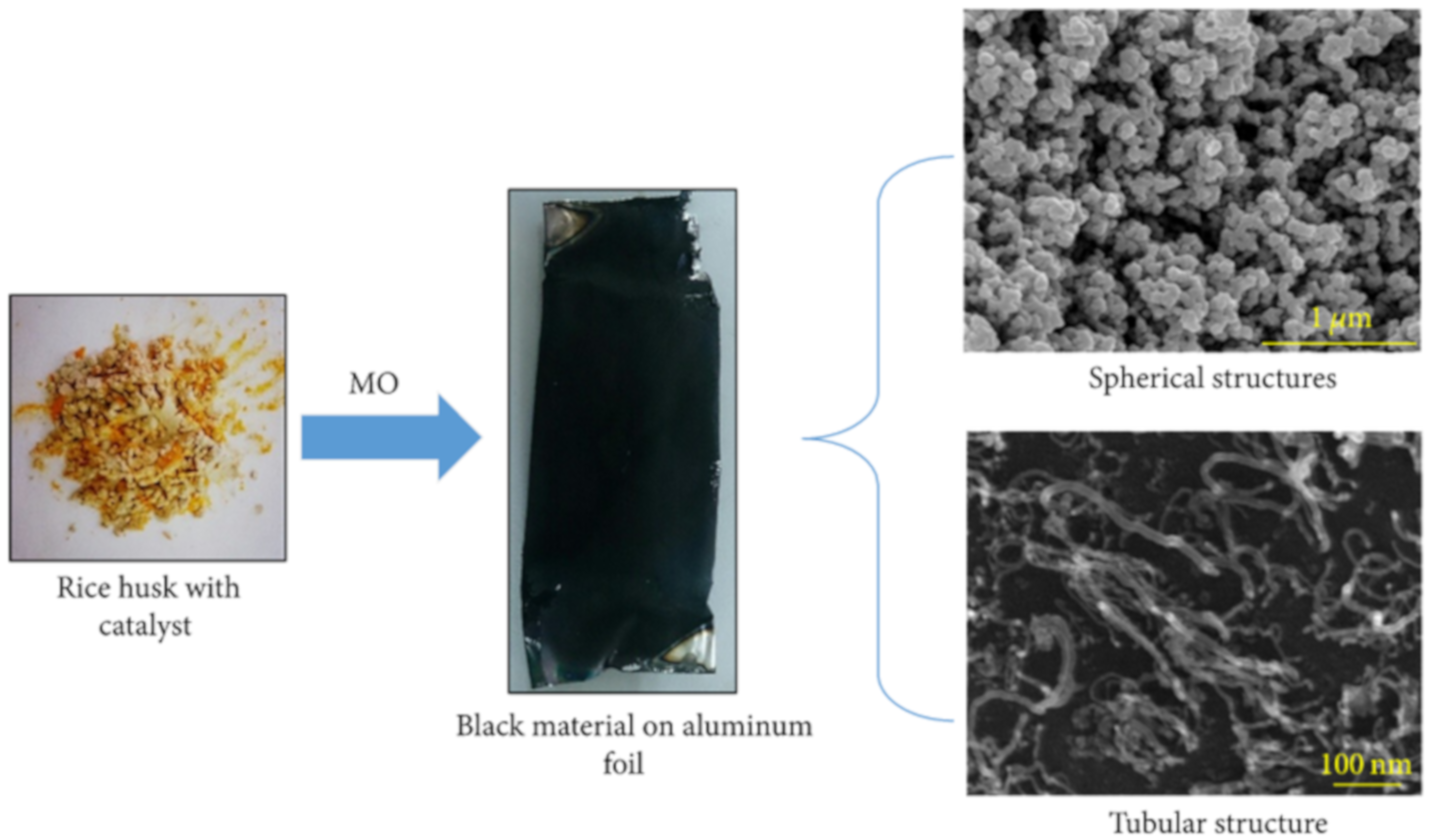






| Biomass Waste | Synthesis Method | Carbon Nanostructure | Application | References |
|---|---|---|---|---|
| Hemp | Hydrothermal/ activation with KOH | Interconnected graphene carbon nanosheets | Supercapacitor | [19] |
| Rice husk | Combustion/chemical activation with KOH | Graphene sheets | Additives in polymer composites, electrochemical performance | [20,21,22] |
| Biochar from Wheat straw | Microwave CVD | MWCNTs | - | [23] |
| Sugarcane Bagasse | Microwave CVD | Graphitic flakes and MWCNTs | - | [24] |
| Rice husk | Microwave CVD | Thin graphene (2–6 layers) MWCNTs | - | [25] |
| Wood sawdust | Conventional Pyrolysis | CNTs | - | [26] |
| Rice straw | Conventional CVD | CNTs | - | [27] |
| Rice residue | Hydrothermal | C-dots | Fe3+ ions and tetracycline detection | [28] |
| Wheat straw | Hydrothermal | C-dots | Labeling, imaging, and sensing | [29] |
| Sugarcane bagasse char | Hydrothermal | C-dots | drug delivery | [30] |
| Rice husk | Pyrolysis | C-dots | Bio-imaging | [31] |
| Rice husk | Hydrothermal, Pyrolysis | GQDs | Fluorescent sensor | [31] |
| Rice grains | Pyrolysis | GQDs | - | [32,33] |
| Rice husk | KOH activation | Graphene oxide | Desalination membrane | [34,35] |
| Rice Straw | Microwave-assisted | Graphene oxide | Ni(II) Adsorption | [36] |
| Rice husk | Pyrolysis | Graphene oxide | - | [37] |
| Carbon Material | Graphite | Graphene | Carbon Nanotube | Fullerene | |
|---|---|---|---|---|---|
| SWCNTs | MWCNTs | ||||
| Dimensions | 3 | 2 | 1 | 1 | 0 |
| Hardness | high | highest | high | high | high |
| Hybridization | sp2 | sp2 | sp2 | sp2 | sp2 |
| Tenacity | Flexible, non-elastic | Flexible, elastic | Elastic | Elastic | Elastic |
| Young modulus | 856.4 ± 0.7(z) 964.0 ± 0.68(a) | 1000 | 1000 | ||
| Thermal conductivity (Wm−1 K−1) | Anisotropic, 1500–2000, 5–10 | 4840–5300 | 3500–6600 | 600–6000 | 0.4 |
| Electrical conductivity | −4000 p, 3.3 c | −2000 | 106–107 | 103–105 | 10−5 |
| Precursor/ Raw Material | Synthesis Method | Reaction Conditions | Chemical Agents | Final Product | Characterization Technique | References |
|---|---|---|---|---|---|---|
| Rice Husk | Chemical Activation | 400–900 °C | KOH | Few-layered graphene | TEM | [65] |
| Rice Straw | Chemical Activation | 700 °C | KOH | Graphene | FESEM, SEM, EDX, AFM, and FETEM | [66] |
| Rice Husk | Chemical Activation | 850 °C/2 h | NaOH, KOH | Multilayered graphene Oxide | FTIR, Raman, SEM, TGA, and TEM | [35] |
| Rice Husk | Microwave-Assisted Synthesis | 650–750 °C | Ethanol, ferrocene catalyst | Graphene | RD, FESEM, FTIR, UV-Vis, Zeta Sizer, and EDX | [67] |
| Rice Bran | Microwave-Assisted Synthesis | 300 °C/15 min | Ferrocene | Graphene Oxide | SEM, HR-TEM, XRD, FTIR, TGA/DTA | [72] |
| Rice Straw | Microwave-Assisted Synthesis | N/A | Ferrocene catalyst | Graphene Oxide | FTIR, SEM, TEM, EDS, Raman | [36] |
| Rice Husk | Pyrolysis | 300–400 °C/10 min, Inert atmosphere | N/A | Graphene Oxide Nanoflakes | XRD, EDS, FESEM, TEM, HR-TEM | [37] |
| Rice Straw | Chemical Vapor Deposition | 800 °C/120 min Heating rate 10 °C/min | Ethanol, ferrocene, or ferrocene nickel nitrate catalysts, camphor substrate, N2 gas | Small outer diameter coiled bundles of CNTs Large outer diameter straight bundles of CNTs- | SEM, TEM, Raman, TGA | [27] |
| Rice Husk | Microwave-Assisted Synthesis | 600 W power, 2.45 GHz frequency/38 min | Ferrocene catalyst, ethanol | Spherical and tubular structures of carbon nanotubes | FESEM | [143] |
| Rice Husk | Microwave-Assisted Synthesis | N/A | H2 and Ar gas | Fiber-like Graphenated carbon nanotubes (g-CNTs) | N/A | [25] |
| Rice Straw | Pyrolysis | 830 °C/60 min | Urea solution, Fe, Ni, and Co catalysts supported on alumina | Carbon nanotubes on Fe-Ni/Al2O3 | TEM, SEM, FTIR | [144] |
| Rice | Thermal Calcination | 400 °C/2 h, N2 atmosphere | N2 atmosphere | Carbon Quantum Dots | HR-TEM, SAED, XRD | [147] |
| Rice Husk | Thermal Calcination | 700 °C/2 h, N2 atmosphere | N/A | Blue luminescent Si-CQDs | N/A | [273] |
| Rice Straw | Microwave Hydrothermal | N/A | Ionic liquid (as a solvent) | Heteroatom-doped carbon dots (IL-CDs) | TEM, UV-Vis, FTIR, AFM | [274] |
| Rice straw | Hydrothermal Carbonization/Acid Oxidation | N/A | HNO3 | Water-Soluble CQDs | N/A | [277] |
| Rice Husk | Hydrothermal Carbonization | 200 °C/6 h | HNO3 | Blue luminescent CDs | FTIR, XPS, DLS, TEM, UV-Vis, Flourescence | [280] |
| Rice Husk | Hydrothermal Carbonization | 190 °C/2 h | EDA, ascorbic acid (functionalizing agents) | Functionalized N-CQDs and CCQDs | FTIR, UV-Vis, HR-TEM, XRD, TGA, XPS | [283] |
| Rice Husk | Hydrothermal Carbonization | 200 °C/10 h, N2 atmosphere | H2SO4, HNO3 | Carbon quantum dot grafted silica nanoparticles (silica–C-NPs) | N/A | [285] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarik, S.; Qureshi, N.; Sattar, Z.; Shaheen, A.; Kalsoom, A.; Imran, M.; Hanif, F. Synthetic Approach to Rice Waste-Derived Carbon-Based Nanomaterials and Their Applications. Nanomanufacturing 2021, 1, 109-159. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1030010
Mubarik S, Qureshi N, Sattar Z, Shaheen A, Kalsoom A, Imran M, Hanif F. Synthetic Approach to Rice Waste-Derived Carbon-Based Nanomaterials and Their Applications. Nanomanufacturing. 2021; 1(3):109-159. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1030010
Chicago/Turabian StyleMubarik, Shamroza, Nawal Qureshi, Zainab Sattar, Aqeela Shaheen, Ambreen Kalsoom, Marryam Imran, and Farzana Hanif. 2021. "Synthetic Approach to Rice Waste-Derived Carbon-Based Nanomaterials and Their Applications" Nanomanufacturing 1, no. 3: 109-159. https://0-doi-org.brum.beds.ac.uk/10.3390/nanomanufacturing1030010






