Oxovanadium(V/IV) Complexes as Redox Mediators for Biofuel Cells: Physical, Magnetic, and Electrochemical Characterization, DFT and Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.1.1. General Information
2.1.2. Synthesis of 1 and 2
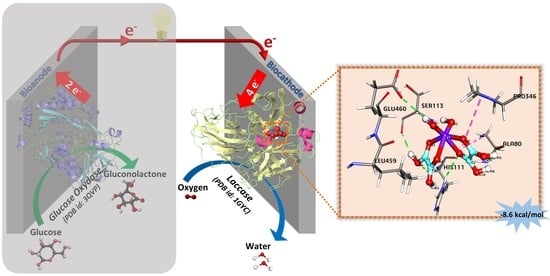
2.1.3. Docking of 1 and 2 with Laccase
2.2. Measurements
2.2.1. Physical Measurements of 1 and 2
2.2.2. Electrochemical Measurements of 1 and 2
2.2.3. Magnetic Measurements of 1 and 2
2.2.4. Electrochemical Measurements of 1 and 2 Docked into Laccase
2.2.5. Magnetic Measurements of 1 and 2 Docked into Laccase
2.2.6. Computational Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Goff, A.; Holzinger, M.; Cosnier, S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell. Mol. Life Sci. 2015, 72, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Elouarzaki, K.; Cheng, D.; Fisher, A.C.; Lee, J.M. Coupling orientation and mediation strategies for efficient electron transfer in hybrid biofuel cells. Nat. Energy 2018, 3, 574–581. [Google Scholar] [CrossRef]
- Sakai, H.; Nakagawa, T.; Tokita, Y.; Hatazawa, T.; Ikeda, T.; Tsujimura, S.; Kano, K. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ. Sci. 2009, 2, 133–138. [Google Scholar] [CrossRef]
- Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Recent Advances in Enzymatic Fuel Cells: Experiments and Modeling. Energies 2010, 3, 803–846. [Google Scholar] [CrossRef] [Green Version]
- Solomon, E.I.; Szilagyi, R.K.; DeBeer George, S.; Basumallick, L. Electronic Structures of Metal Sites in Proteins and Models: Contributions to Function in Blue Copper Proteins. Chem. Rev. 2004, 104, 419–458. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-Dimensional Graphene-Carbon Nanotube Hybrid for High-Performance Enzymatic Biofuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef]
- Neto, S.A.; Forti, J.C.; Andrade, A.R. De An Overview of Enzymatic Biofuel Cells. Electrocatalysis 2010, 1, 87–94. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Sunaga, N.; Akitsu, T. Anthraquinone and L-amino Acid Derivatives Schiff Base Cu(II) Complexes as a Mediator between Cathode of Biofuel Cell and Oxygen-reducing Laccase Abstract. Trends Green Chem. 2017, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.; Chatterjee, S.; Si, T.K.; Mukherjea, K.K. Synthesis, structural characterization, VHPO mimicking peroxidative bromination and DNA nuclease activity of oxovanadium(v) complexes. Dalton Trans. 2013, 42, 13425–13435. [Google Scholar] [CrossRef]
- Ando, R.; Mori, S.; Hayashi, M.; Yagyu, T.; Maeda, M. Structural characterization of pentadentate salen-type Schiff-base complexes of oxovanadium (IV) and their use in sulfide oxidation. Inorg. Chim. Acta 2004, 357, 1177–1184. [Google Scholar] [CrossRef]
- Maeda, Y.; Kakiuchi, N.; Matsumura, S.; Nishimura, T.; Kawamura, T.; Uemura, S. Oxovanadium complex-catalyzed aerobic oxidation of propargylic alcohols. J. Org. Chem. 2002, 67, 6718–6724. [Google Scholar] [CrossRef]
- Shen, Y.; Atobe, M.; Li, W.; Nonaka, T. Paired electrosynthesis of epoxides and dibromides from olefinic compounds. Electrochim. Acta 2003, 48, 1041–1046. [Google Scholar] [CrossRef]
- Sakurai, H.; Tsuchiya, K. A biomimetic model for vanadium-containing bromoperoxidase. FEBS Lett. 1990, 260, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.; Carrano, C.J. Coordination chemistry of vanadium in biological systems. Coord. Chem. Rev. 1991, 109, 61–105. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Akitsu, T. Anthraquinone Derivative Chiral Schiff Base Copper(II) Complexes for Enzyme Type Bio-Fuel Cell Mediators. J. Electr. Eng. 2016, 4, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Mitsumoto, Y.; Sunaga, N.; Akitsu, T. Polarized light induced molecular orientation in laccase for chiral azo- salen Mn(II), Co(II), Ni(II), Cu(II), Zn(II) mediators toward application for biofuel cell. Scifed J. Chem. Res. 2017, 1, 1–12. [Google Scholar]
- Sano, A.; Yagi, S.; Haraguchi, T.; Akitsu, T. Synthesis of MnII and CuII complexes including azobenzene and its application to mediators of laccase for biofuel cells. J. Indian Chem. Soc. 2018, 95, 487–494. [Google Scholar]
- Kunitake, F.; Kim, J.; Yagi, S.; Yamzaki, S.; Haraguchi, T.; Akitsu, T. Chiral Recognition of Azo-Schi ff Base Ligands, Their Cu(II) Complexes, and Their Docking to Laccase as Mediators. Symmetry 2019, 11, 666. [Google Scholar] [CrossRef] [Green Version]
- Kajiwara, K.; Yamane, S.; Haraguchi, T.; Pradhan, S.; Sinha, C.; Giri, S.; Roymahaptra, G.; Moon, D.; Akitsu, T. Computational Design of Azo-anthraquinone Schiff Base Mn Complexes as Mediators for Biofuel Cell Cathode. J. Chem. Chem. Eng. 2019, 13, 23–33. [Google Scholar] [CrossRef]
- Sehimi, H.; Chérif, I.; Zid, M.F. Crystal structure of bis[4-(dimethylamino)-pyridinium] aquabis(oxalato)oxidovanadate(IV)dihydrate. Acta Crystallogr. Sect. E 2016, 72, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Sehimi, H.; Akitsu, T.; Zid, M.F. Synthesis and structural study of tris(2,6-diaminopyridinium) bis(oxalato)dioxidovanadate(V) 2.5-hydrate. Acta Crystallogr. Sect. E 2019, 75, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Kominato, C.; Akitsu, T. Photoinduced molecular orientation of catalytic-like chiral azo-schiff base complexes in schiff base complexes in PMMA or laccase matrices. Lett. Appl. Nanobioscience 2015, 4, 264–270. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009; p. 201. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity-A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.K.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Sehimi, H.; Essghaier, B.; Barea, E.; Sadfi-Zouaoui, N.; Zid, M.F. Synthesis, structural study, magnetic susceptibility and antimicrobial activity of the first (μ-oxo)-bis(oxalato)-vanadium(IV) 1D coordination polymer. J. Mol. Struct. 2019, 1175, 865–873. [Google Scholar] [CrossRef]
- Ben Moussa, O.; Chebbi, H.; Zid, M.F. Synthesis, crystal structure, vibrational study, optical properties and Hirshfeld surface analysis of bis(2,6-diaminopyridinium) tetrachloridocobaltate(II) monohydrate. J. Mol. Struct. 2019, 1180, 72–80. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Sakurai, T.; Monjushiro, H.; Takeda, S. Magnetic studies of the trinuclear center in laccase and ascorbate oxidase approached by EPR spectroscopy and magnetic susceptibility measurements. Biochim. Biophys. Acta 1998, 1384, 160–170. [Google Scholar] [CrossRef]











| Ion Pair of 1 | Ion Pair of 1 [32] (as Previously Reported) | Ion Pair of 2 | Attribution |
|---|---|---|---|
| 3459, 2941 | 3482, 3373, 2935 | 3336, 2919 | ν(C-H); ν(N-H) |
| 3162, 3102 | 3229, 3082 | 3196 | νs(O-H); νas(O-H) |
| - | - | 2363 | ν(N-H...O) |
| 1644 | 1639 | 1654 | νas(COO) |
| 1561 | 1559 | - | ν(C=C) |
| 1382 | 1397 | 1398 | νs(COO) |
| - | - | 1297 | ν(C-NH2) |
| 1253, 1212 | 1236, 1212 | 1170,1121 | ν(C-N) |
| 1068 | 1061 | - | δ(O-H) |
| 994 | 951 | 964 | ν(V=O) |
| 891 | 891 | - | δ(N-H) |
| 808 | 802 | 800 | γ(C-N); γ(C-C) |
| 552, 479 | 530, 478 | 537 | ν(V-O) |
| Bond Name | Distance (Å) | Bond Category | Bond Type |
|---|---|---|---|
| C2:H6—A:ALA80:O | 1.94933 | Hydrogen bond | Conventional hydrogen bond |
| C2:H5—A:ALA80:O | 1.98495 | Hydrogen bond | Conventional hydrogen bond |
| C2:H4—A:SER113:O | 2.14896 | Hydrogen bond | Conventional hydrogen bond |
| A:HIS111:HD1—C2:O7 | 2.65241 | Hydrogen bond | Conventional hydrogen bond |
| C2:H1—A:GLU460:OE1 | 2.65976 | Hydrogen bond | Conventional hydrogen bond |
| A:HIS111:HA—C2:O6 | 2.74924 | Hydrogen bond | Carbon hydrogen bond |
| A:ALA80—C2 | 4.35591 | Hydrophobic | Pi-Alkyl |
| A:PRO346—C2 | 5.29772 | Hydrophobic | Pi-Alkyl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsuumi, N.; Sehimi, H.; Pradhan, S.; Kim, S.; Haraguchi, T.; Akitsu, T. Oxovanadium(V/IV) Complexes as Redox Mediators for Biofuel Cells: Physical, Magnetic, and Electrochemical Characterization, DFT and Molecular Docking. Compounds 2021, 1, 15-28. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds1010003
Katsuumi N, Sehimi H, Pradhan S, Kim S, Haraguchi T, Akitsu T. Oxovanadium(V/IV) Complexes as Redox Mediators for Biofuel Cells: Physical, Magnetic, and Electrochemical Characterization, DFT and Molecular Docking. Compounds. 2021; 1(1):15-28. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds1010003
Chicago/Turabian StyleKatsuumi, Natsuki, Hiba Sehimi, Sayantan Pradhan, Sanyobi Kim, Tomoyuki Haraguchi, and Takashiro Akitsu. 2021. "Oxovanadium(V/IV) Complexes as Redox Mediators for Biofuel Cells: Physical, Magnetic, and Electrochemical Characterization, DFT and Molecular Docking" Compounds 1, no. 1: 15-28. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds1010003