How Threatened Is Scincella huanrenensis? An Update on Threats and Trends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Introduction
2.2. Data Collection
2.3. Range Modeling
2.4. Estimating the Extent of Occurence
2.5. Assessment
2.6. General Threats to the Species
2.7. Species Assessment
2.7.1. Population Size Reduction
2.7.2. Geographic Range
2.7.3. Small Population Size and Decline
2.7.4. Very Small or Restricted Population
2.7.5. Quantitative Analysis
3. Results
3.1. Habitat Suitability Modeling
3.2. Geographic Range Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wren, S.; Angulo, A.; Meredith, H.; Kielgast, J.; Dos Santos, M.; Bishop, P.; IUCN SSC Amphibian Specialist Group. Amphibian Conservation Action Plan. 2015. Available online: http://www.amphibians.org/acap/ (accessed on 12 December 2020).
- Rodrigues, A.S.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Al Dhaheri, S.; Bennett, E.L.; Biggs, D.; Bignell, A.; Byers, O.; Cooney, R.; Donaldson, J.; Dublin, H.T.; Eggermont, H.; et al. IUCN’s encounter with 007: Safeguarding consensus for conservation. Oryx 2019, 53, 741–747. [Google Scholar] [CrossRef]
- Rondinini, C.; Di Marco, M.; Visconti, P.; Butchart, S.H.; Boitani, L. Update or Outdate: Long-Term Viability of the IUCN Red List. Conserv. Lett. 2013, 7, 126–130. [Google Scholar] [CrossRef]
- Betts, J.; Young, R.P.; Hilton-Taylor, C.; Hoffmann, M.; Rodríguez, J.P.; Stuart, S.N.; Milner-Gulland, E. A framework for evaluating the impact of the IUCN Red List of threatened species. Conserv. Biol. 2019, 34, 632–643. [Google Scholar] [CrossRef]
- Canseco-Márquez, L.; Ramírez-González, C.G.; González-Bernal, E. Discovery of another new species of Charadrahyla (Anura, Hylidae) from the cloud forest of northern Oaxaca, México. Zootaxa 2017, 4329, 64–72. [Google Scholar] [CrossRef] [PubMed]
- IUCN SSC Amphibian Specialist Group. Sarcohyla celata. In The IUCN Red List of Threatened Species; IUCN SSC Amphibian Specialist Group: Gland, Switzerland, 2020; Volume T55438A53953583. [Google Scholar]
- Broderick, A.C.; Frauenstein, R.; Glen, F.; Hays, G.C.; Jackson, A.L.; Pelembe, T.; Ruxton, G.D.; Godley, B.J. Are green turtles globally endangered? Glob. Ecol. Biogeogr. 2006, 15, 21–26. [Google Scholar] [CrossRef]
- Seminoff, J.A.; Shanker, K. Marine turtles and IUCN Red Listing: A review of the process, the pitfalls, and novel assessment approaches. J. Exp. Mar. Biol. Ecol. 2008, 356, 52–68. [Google Scholar] [CrossRef]
- Zhao, W.; Li, P. Scincella huanrenensis. In The IUCN Red List of Threatened Species; IUCN SSC Amphibian Specialist Group: Gland, Switzerland, 2019. [Google Scholar]
- NIBR. Red Data Book of Republic of Korea. In Volume 2: Amphibians and Reptiles; National Institute of Biological Resources, Ministry of Environment: Incheon, Korea, 2019. [Google Scholar]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. 2019. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 15 February 2021).
- Zhao, E.; Huang, K. A survey of amphibians and reptiles in Liaoning province. Acta Herpetol. Sin. 1982, 1, 1–23. [Google Scholar]
- Dong, B.-J.; Wang, J.-Q.; Zhou, Z.-Y.; Lu, Y.-Y.; Li, P.-P. Research on reproductive behavior and strategy of Scincella huanrensis. Sichuan J. Zool. 2007, 26, 302–304. [Google Scholar]
- Lee, S.-C. Systematic and Ecological Studies of the Suborder Sauria (Reptilia, Squamata) in Korea; University of Incheon: Incheon, Korea, 2010. [Google Scholar]
- Chang, M.; Song, J.; Lee, J.; Oh, H. The current status of Korean lizards (Reptilia: Squamata). Korean J. Environ. Ecol. 2006, 20, 352–358. [Google Scholar]
- Song, J.Y. Current status and distribution of reptiles in the Republic of Korea. Korean J. Environ. Biol. 2007, 25, 124–138. [Google Scholar]
- Koo, K.S.; Kwon, S.-R.; Chang, M.-H.; Song, J.-Y. New geographic distribution record of the rare skink Scincella huanrenensis Zhao and Huang, 1982 (Squamata, Scincidae). Herpetol. Notes 2020, 13, 199–201. [Google Scholar]
- Zhao, E.-M.; Zhao, K.-T.; Zhou, K.-Y. Fauna Sinica, Reptilia Vol 2, Squamata, Lacertilia; Science Press: Beijing, China, 1999. [Google Scholar]
- Won, H.K. Amphibian and Reptilian fauna of Korea; Academy of Sciences: Pyongyang, Democratic People’s Republic of Korea, 1971; p. 170. [Google Scholar]
- Kim, L.; Han, G. Chosun Animal Encyclopedia, Herpetology Volume; Roh, H., Ed.; Science and Technology Publisher: Pyongyang, Democratic People’s Republic of Korea, 2009; p. 138. [Google Scholar]
- Dong, B.-J. Current status and protection of Scincella huanrenensis. Sichuan J. Zool. 2005, 24, 279–281. [Google Scholar]
- Shin, Y.; Jang, Y.; Allain, S.J.R.; Borzée, A. Catalogue of herpetological specimens of the Ewha Womans University Natural History Museum (EWNHM), Republic of Korea. ZooKeys 2020, 965, 103–139. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Koo, K.-S.; Kim, I.-H.; Park, D. Complete mitochondrial genomes of Scincella vandenburghi and S. huanrenensis (Squamata: Scincidae). Mitochondrial DNA Part B 2016, 1, 237–238. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, S.; Kim, M.; Shin, M.; Yoon, H.; Kim, K. EcoBank: A flexible database platform for sharing ecological data. Biodivers. Data J. 2021, 9, e61866. [Google Scholar] [CrossRef]
- Bobrov, V.V. North, west, and east limits of the Sahara-Gobi faunistic region in Eurasia (according to distribution of lizards (reptilia, sauria). Biol. Bull. 1999, 26, 469–478. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Tuanmu, M.-N.; Jetz, W. A global 1-km consensus land-cover product for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr. 2014, 23, 1031–1045. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Ofori, B.Y.; Stow, A.J.; Baumgartner, J.B.; Beaumont, L.J. Combining dispersal, landscape connectivity and habitat suitability to assess climate-induced changes in the distribution of Cunningham’s skink, Egernia cunninghami. PLoS ONE 2017, 12, e0184193. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for statistical computing: Vienna, Austria, 2020. [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.0-7. 2019. Available online: https://CRAN.R-project.org/package=raster (accessed on 23 February 2021).
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the ’Geospatial’ Data Abstraction Library. R Package Version 1.4-8. 2019. Available online: https://CRAN.R-project.org/package=rgdal (accessed on 23 February 2021).
- Bivand, R.; Rundell, C. Rgeos: Interface to Geometry Engine—Open Source (’GEOS’). R Package Version 0.5-2. 2019. Available online: https://CRAN.R-project.org/package=rgeos (accessed on 23 February 2021).
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Peterson, A.T.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. Ntbox: An R package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.1-4. 2017. Available online: https://CRAN.R-project.org/package=dismo (accessed on 23 February 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.4.6. 2020. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 23 February 2021).
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Dauby, G. ConR: Computation of Parameters Used in Preliminary Assessment of Conservation Status. R Package Version 1.3.0. 2020. Available online: https://CRAN.R-project.org/package=ConR (accessed on 23 February 2021).
- R Core Team. R Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 23 February 2021).
- Maes, D.; Isaac, N.J.B.; Harrower, C.A.; Collen, B.; Van Strien, A.J.; Roy, D.B. The use of opportunistic data for IUCN Red List assessments. Biol. J. Linn. Soc. 2015, 115, 690–706. [Google Scholar] [CrossRef]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akcakaya, H.T.; Leader-Williams, N.; Miller-Gulland, E.J.; Stuartt, S.N. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Mace, G.M.; Kunin, W. Classifying threatened species: Means and ends. Philos. Trans. R. Soc. B Biol. Sci. 1994, 344, 91–97. [Google Scholar] [CrossRef]
- Hermoso, V.; Kennard, M.J.; Linke, S. Evaluating the costs and benefits of systematic data acquisition for conservation assessments. Ecography 2014, 38, 283–292. [Google Scholar] [CrossRef]
- Macias, D.; Shin, Y.; Borzée, A. An update on the conservation status and ecology of Korean terrestrial squamates. J. Nat. Conserv. 2021, 60, 125971. [Google Scholar] [CrossRef]
- Yang, C.; Tang, S.; Luo, Z. Distribution changes of Chinese skink (Eumeces chinensis) in China: The impacts of global climate change. Asian Herpetol. Res. 2020, 11, 132–139. [Google Scholar]
- COP21. Paris Climate Agreement; United Nations: Paris, France, 2015; Chapter 27. [Google Scholar]
- Mundaca, L.; Ürge-Vorsatz, D.; Wilson, C. Demand-side approaches for limiting global warming to 1.5 °C. Energy Effic. 2019, 12, 343–362. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Moss, R.H.; Babkier, M.; Brinkman, S.; Calvo, E.; Carter, T.; Edmonds, J.A.; Elgizouli, I.; Emori, S.; Lin, E.; Hibbbard, L.; et al. Towards New Scenarios for the Analysis of Emissions: Climate Change, Impacts and Response Strategies; Intergovernmental Panel on Climate Change, Ed.; Intergovernmental Panel on Climate Change Secretariat (IPCC): Noordwijkerhout, The Netherlands, 2008. [Google Scholar]
- Xu, Y.; Zhou, B.T.; Wu, J.; Han, Z.Y.; Zhang, Y.X.; Wu, J. Asian climate change under 1.5–4 C warming targets. Adv. Clim. Chang. Res. 2017, 8, 99–107. [Google Scholar] [CrossRef]
- Kim, I.H.; Jang, H.J.; Roh, N.H.; Park, D.S. Endangered risk analysis of Korean three rare reptile species. In Proceedings of the 8th Annual Meeting of the Society of Korean Herpetologists, Wonju, Korea, 10 July 2015. [Google Scholar]
- Stone, R. Seeking Cures for North Korea’s Environmental Ills. Science 2012, 335, 1425–1426. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-D.; Miller-Rushing, A.J. Degradation, urbanization, and restoration: A review of the challenges and future of conservation on the Korean Peninsula. Biol. Conserv. 2014, 176, 262–276. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, D.I.; Jang, M.H. Distribution of reptiles in South Korea—Based on the 3rd National Ecosystem Survey. Korean J. Herpetol. 2016, 7, 30–35. [Google Scholar]
- Aubry, K.B.; Raley, C.M.; McKelvey, K.S. The importance of data quality for generating reliable distribution models for rare, elusive, and cryptic species. PLoS ONE 2017, 12, e0179152. [Google Scholar] [CrossRef]
- Arrondo, E.; Moleón, M.; Cortés-Avizanda, A.; Jiménez, J.; Beja, P.; Sánchez-Zapata, J.A.; Donázar, J.A. Invisible barriers: Differential sanitary regulations constrain vulture movements across country borders. Biol. Conserv. 2018, 219, 46–52. [Google Scholar] [CrossRef]
- National Biodiversity Strategy and Action Plan. In National Biodiversity Strategy and Action Plan of DPR Korea; Gef, U. (Ed.) UNEP & GEF: Pyongyang, Democratic People’s Republic of Korea, 2017; Available online: http://extwprlegs1.fao.org/docs/pdf/prk163532.pdf (accessed on 12 February 2021).
- Cook, C.N.; Mascia, M.B.; Schwartz, M.W.; Possingham, H.P.; Fuller, R.A. Achieving conservation science that bridges the knowledge–action boundary. Conserv. Biol. 2013, 27, 669–678. [Google Scholar] [CrossRef]


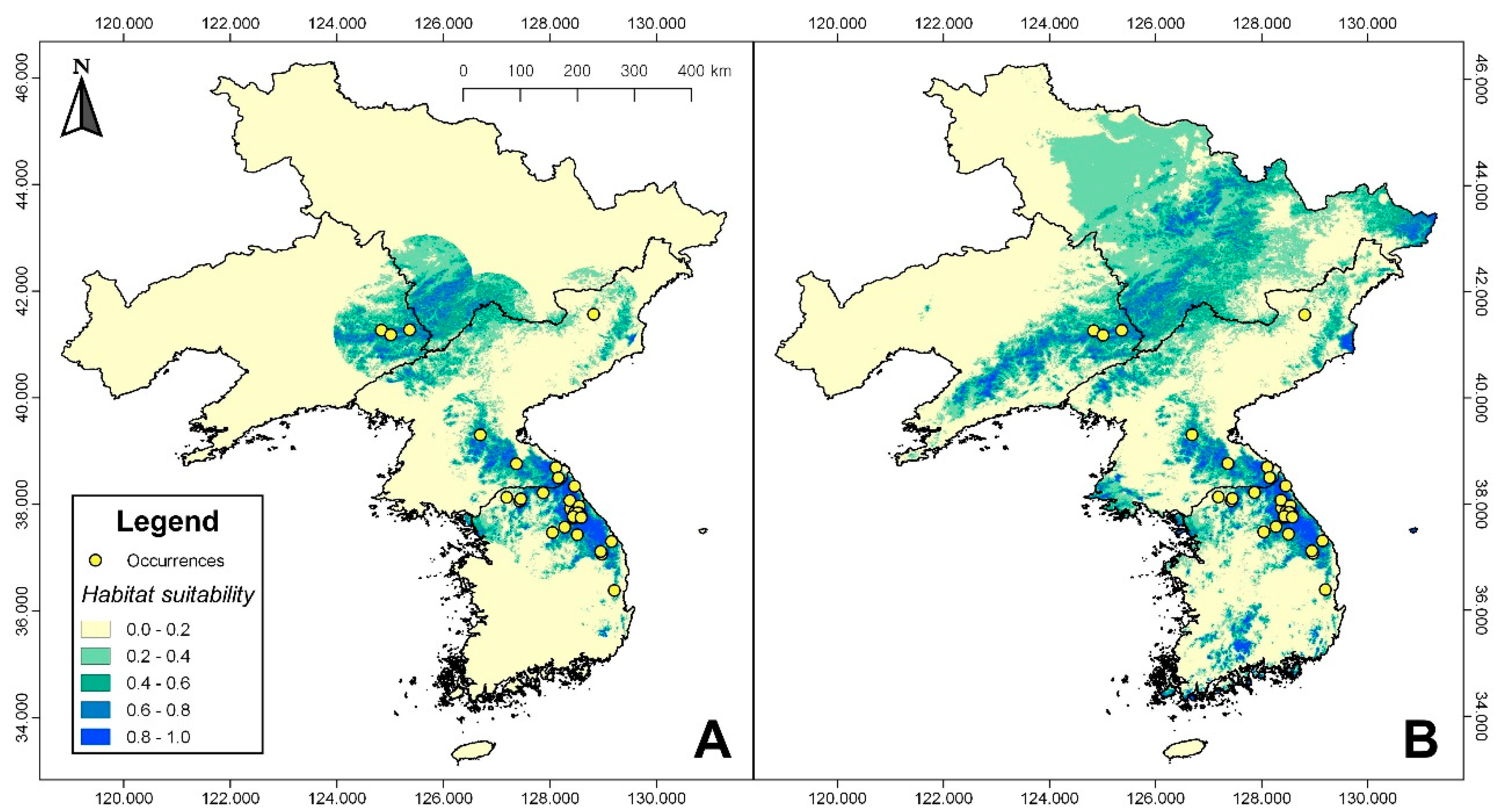
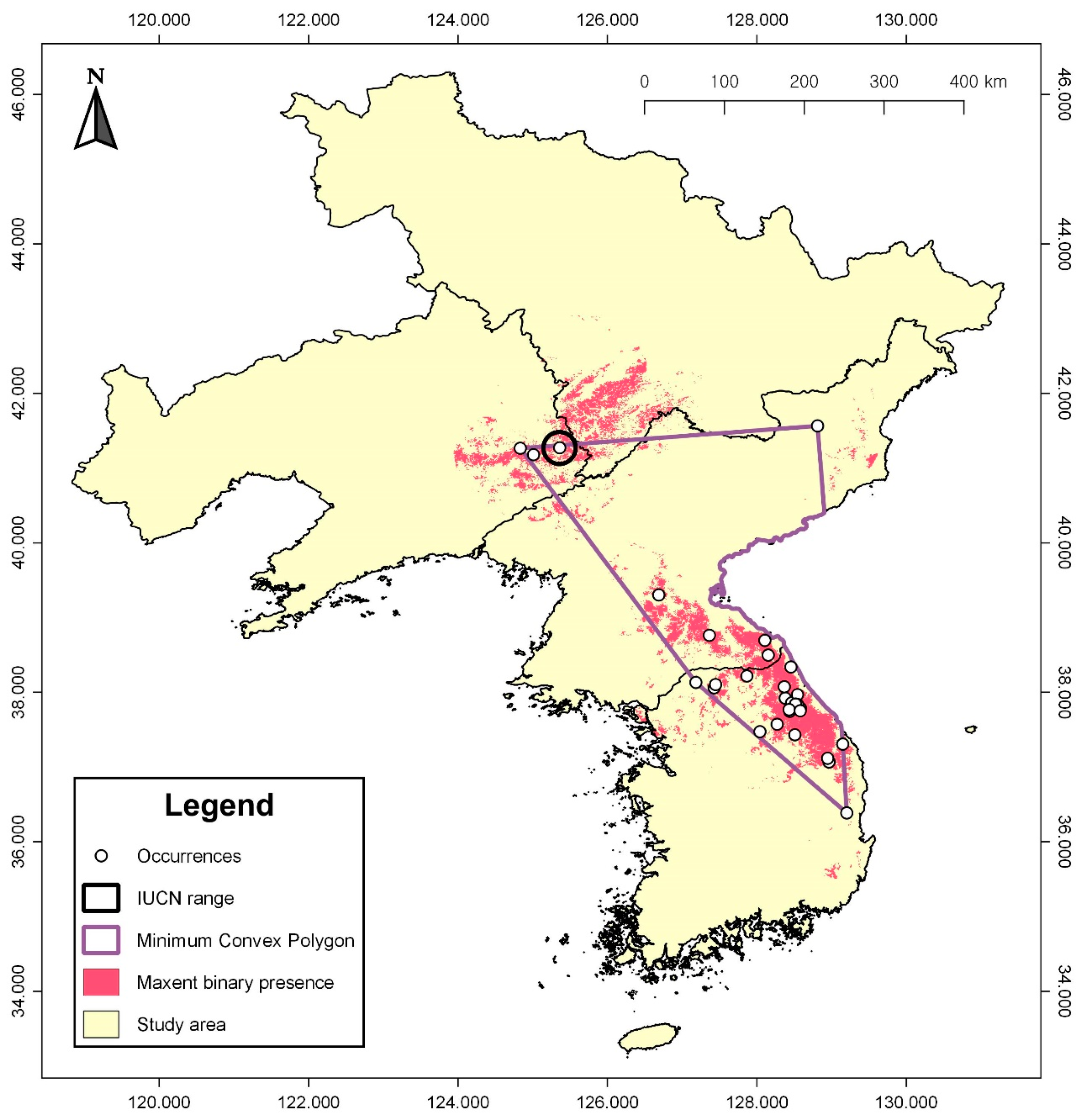
| Origin | Latitude (° N) | Longitude (° E) |
|---|---|---|
| Field survey | 38.07 | 128.36 |
| Field survey | 37.43 | 128.50 |
| Field survey | 36.38 | 129.20 |
| Field survey | 37.83 | 128.54 |
| Field survey | 37.83 | 128.53 |
| Field survey | 36.38 | 129.20 |
| Field survey | 37.76 | 128.57 |
| Field survey | 37.92 | 128.37 |
| Field survey | 38.13 | 127.18 |
| Field survey | 38.06 | 127.42 |
| Field survey | 38.10 | 127.44 |
| Field survey | 37.06 | 128.96 |
| Field survey | 37.74 | 128.43 |
| Field survey | 37.76 | 128.57 |
| Field survey | 37.85 | 128.46 |
| Field survey | 37.11 | 128.94 |
| Field survey | 36.38 | 129.20 |
| Field survey | 38.07 | 128.36 |
| Field survey | 37.57 | 128.27 |
| Field survey | 37.78 | 128.58 |
| Field survey | 37.47 | 128.04 |
| EWNHM-ANIMAL | 37.96 | 128.54 |
| iNaturalist | 37.74 | 128.43 |
| iNaturalist | 37.83 | 128.54 |
| iNaturalist | 37.83 | 128.54 |
| iNaturalist | 37.84 | 128.51 |
| GBIF | 37.76 | 128.43 |
| GBIF | 37.75 | 128.58 |
| NIE | 38.21 | 127.86 |
| NIE | 38.33 | 128.45 |
| NIE | 37.30 | 129.14 |
| Dong 2005 | 41.26 | 124.83 |
| Zhao and Huang, 1982 | 41.18 | 125.01 |
| Dong 2008 | 41.26 | 124.83 |
| Bobrov 1999 * | 42.16 | 125.66 |
| Workshop * | 38.36 | 128.16 |
| Won 1971/Kim and Han 2009 | 38.76 | 127.36 |
| Won 1971/Kim and Han 2009 | 39.30 | 126.68 |
| Won 1971/Kim and Han 2009 | 41.56 | 128.81 |
| Won 1971/Kim and Han 2009 | 38.69 | 128.10 |
| Won 1971/Kim and Han 2009 | 38.49 | 128.15 |
| IUCN | 41.27 | 125.36 |
| Variables | Contribution (%) | Permutation Importance |
|---|---|---|
| Elevation | 27 | 30.3 |
| Isothermality (bio3) | 21.7 | 14.9 |
| Precipitation of driest quarter (bio17) | 21 | 41.7 |
| Deciduous broadleaf forest cover | 16.7 | 1.5 |
| Annual precipitation (bio12) | 7.9 | 9.5 |
| Mixed forest cover | 4.5 | 1.6 |
| Needleleaf forest cover | 1.1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.; Messenger, K.R.; Koo, K.S.; Lee, S.C.; Hou, M.; Borzée, A. How Threatened Is Scincella huanrenensis? An Update on Threats and Trends. Conservation 2021, 1, 58-72. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1010005
Shin Y, Messenger KR, Koo KS, Lee SC, Hou M, Borzée A. How Threatened Is Scincella huanrenensis? An Update on Threats and Trends. Conservation. 2021; 1(1):58-72. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1010005
Chicago/Turabian StyleShin, Yucheol, Kevin R. Messenger, Kyo Soung Koo, Sang Cheol Lee, Mian Hou, and Amaël Borzée. 2021. "How Threatened Is Scincella huanrenensis? An Update on Threats and Trends" Conservation 1, no. 1: 58-72. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1010005







