New Online Resource on the 3Rs Principles of Animal Research for Wildlife Biologists, Ecologists, and Conservation Managers
Abstract
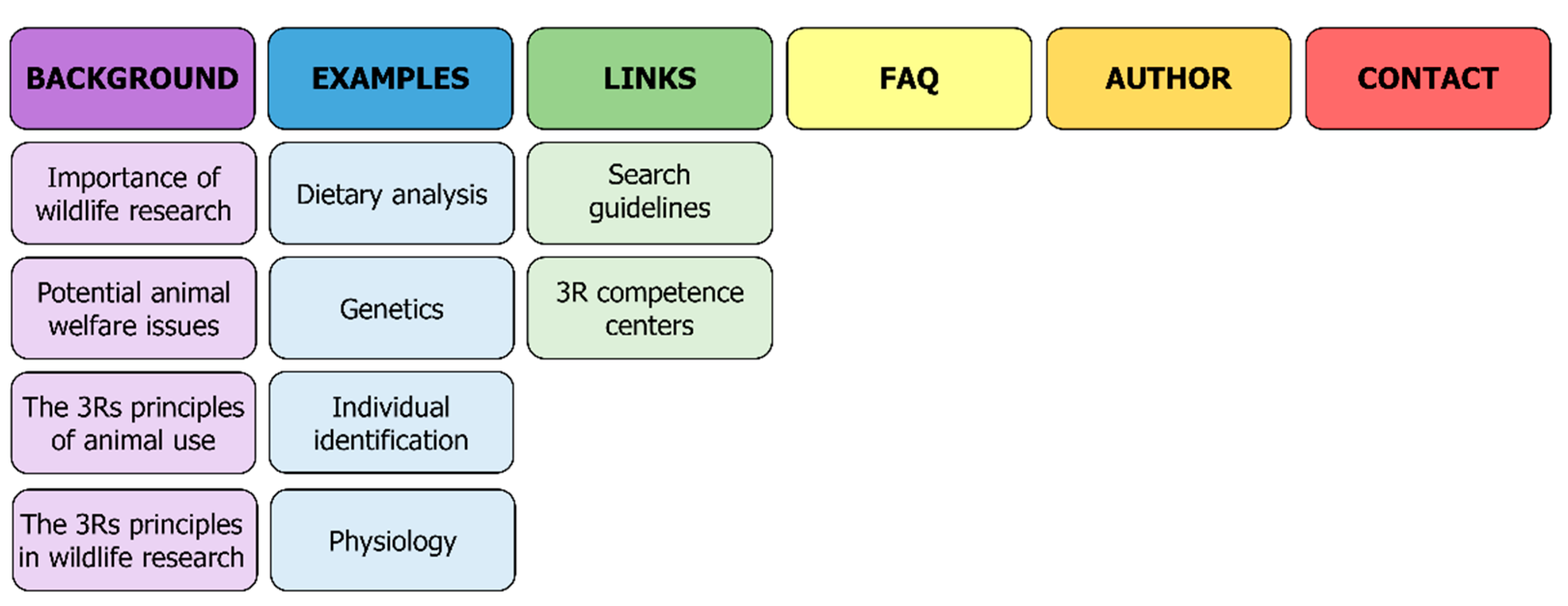
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tittensor, D.P.; Walpole, M.; Hill, S.L.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.M.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241. [Google Scholar] [CrossRef]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Galetti, M.; Alamgir, M.; Crist, E.; Mahmoud, M.I.; Laurance, W.F. World scientists’ warning to humanity: A second notice. Bioscience 2017, 67, 1026–1028. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Union for Conservation of Nature. The Red List of Threatened Species; IUCN: Cambridge, UK, 2019; Available online: http://www.iucnredlist.org (accessed on 9 February 2021).
- Visconti, P.; Pressey, R.L.; Giorgini, D.; Maiorano, L.; Bakkenes, M.; Boitani, L.; Alkemade, R.; Falcucci, A.; Chiozza, F.; Rondinini, C. Future hotspots of terrestrial mammal loss. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2693–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desurmont, G.A.; Zemanova, M.A.; Turlings, T.C.J. The gastropod menace: Slugs on Brassica plants affect caterpillar survival through consumption and interference with parasitoid attraction. J. Chem. Ecol. 2016, 42, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.J.; Koprowski, J.L. Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 2017, 209, 34–44. [Google Scholar] [CrossRef]
- Zemanova, M.A.; Knop, E.; Heckel, G. Introgressive replacement of natives by invading Arion pest slugs. Sci. Rep. 2017, 7, 14908. [Google Scholar] [CrossRef] [Green Version]
- Kharouba, H.M.; Ehrlén, J.; Gelman, A.; Bolmgren, K.; Allen, J.M.; Travers, S.E.; Wolkovich, E.M. Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl. Acad. Sci. USA 2018, 115, 5211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemanova, M.A.; Perotto-Baldivieso, H.L.; Dickins, E.L.; Gill, A.B.; Leonard, J.P.; Wester, D.B. Impact of deforestation on habitat connectivity thresholds for large carnivores in tropical forests. Ecol. Process. 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Zemanova, M.A.; Broennimann, O.; Guisan, A.; Knop, E.; Heckel, G. Slimy invasion: Climatic niche and current and future biogeography of Arion slug invaders. Divers. Distrib. 2018, 24, 1627–1640. [Google Scholar] [CrossRef] [Green Version]
- Doherty, T.S.; Balouch, S.; Bell, K.; Burns, T.J.; Feldman, A.; Fist, C.; Garvey, T.F.; Jessop, T.S.; Meiri, S.; Driscoll, D.A. Reptile responses to anthropogenic habitat modification: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1265–1279. [Google Scholar] [CrossRef]
- Williams, D.R.; Balmford, A.; Wilcove, D.S. The past and future role of conservation science in saving biodiversity. Conserv. Lett. 2020, 13, e12720. [Google Scholar] [CrossRef] [Green Version]
- Brister, E.; Holbrook, J.B.; Palmer, M.J. Conservation science and the ethos of restraint. Conserv. Sci. Pract. 2021, 3, e381. [Google Scholar]
- Fonseca, C.R.; Paterno, G.B.; Guadagnin, D.L.; Venticinque, E.M.; Overbeck, G.E.; Ganade, G.; Metzger, J.P.; Kollmann, J.; Sauer, J.; Cardoso, M.Z.; et al. Conservation biology: Four decades of problem-and solution-based research. Perspect. Ecol. Conserv. 2021, 19, 121–130. [Google Scholar]
- Shrestha, Y.; Lapeyre, R. Modern wildlife monitoring technologies: Conservationists versus communities? A case study: The Terai-Arc Landscape, Nepal. Conserv. Soc. 2018, 16, 91–101. [Google Scholar] [CrossRef]
- Chen, R.; Little, R.; Mihaylova, L.; Delahay, R.; Cox, R. Wildlife surveillance using deep learning methods. Ecol. Evol. 2019, 9, 9453–9466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Thaden, A.; Nowak, C.; Tiesmeyer, A.; Reiners, T.E.; Alves, P.C.; Lyons, L.A.; Mattucci, F.; Randi, E.; Cragnolini, M.; Galián, J. Applying genomic data in wildlife monitoring: Development guidelines for genotyping degraded samples with reduced single nucleotide polymorphism panels. Mol. Ecol. Resour. 2020, 20, 662–680. [Google Scholar] [CrossRef] [Green Version]
- European Commission. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017; EC: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1581689520921&uri=CELEX:52020DC0016 (accessed on 18 April 2021).
- Soulé, M.E. What is conservation biology? Bioscience 1985, 35, 727–734. [Google Scholar]
- Jewell, Z. Effect of monitoring technique on quality of conservation science. Conserv. Biol. 2013, 27, 501–508. [Google Scholar] [CrossRef]
- Costello, M.J.; Beard, K.H.; Corlett, R.T.; Cumming, G.S.; Devictor, V.; Loyola, R.; Maas, B.; Miller-Rushing, A.J.; Pakeman, R.; Primack, R.B. Field work ethics in biological research. Biol. Conserv. 2016, 203, 268–271. [Google Scholar] [CrossRef]
- Zemanova, M.A. Towards more compassionate wildlife research through the 3Rs principles: Moving from invasive to non-invasive methods. Wildl. Biol. 2020, 2020, wlb.00623. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.B.; Brown, C.R. Blood sampling reduces annual survival in cliff swallows (Petrochelidon pyrrhonota). Auk 2009, 126, 853–861. [Google Scholar] [CrossRef]
- Durrer, H.; Golay, N. Inflammation due to toe-clipping in natterjack toads (Bufo calamita). Amphibia-Reptilia 1994, 15, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Phillott, A.D.; McDonald, K.R.; Skerratt, L.F. Inflammation in digits of unmarked and toe-tipped wild hylids. Wildl. Res. 2011, 38, 204–207. [Google Scholar] [CrossRef]
- Bloch, N.; Irschick, D.J. Toe-clipping dramatically reduces clinging performance in a pad-bearing lizard (Anolis carolinensis). J. Herpetol. 2005, 39, 288–293. [Google Scholar] [CrossRef]
- Schmidt, K.; Schwarzkopf, L. Visible implant elastomer tagging and toe-clipping: Effects of marking on locomotor performance of frogs and skinks. Herpetol. J. 2010, 20, 99–105. [Google Scholar]
- Olivera-Tlahuel, C.; Perez-Mendoza, H.A.; Zuniga-Vega, J.J.; Rubio-Rocha, L.C.; Bock, B.C.; Rojas-Gonzalez, R.I.; Zamora-Abrego, J.G.; Alzate, E.; Ortega-Leon, A.M.; Maceda-Cruz, R.J.; et al. Effect of toe-clipping on the survival of several lizard species. Herpetol. J. 2017, 27, 266–275. [Google Scholar]
- Field, I.C.; Harcourt, R.G.; Boehme, L.; de Bruyn, P.J.N.; Charrassin, J.B.; McMahon, C.R.; Bester, M.N.; Fedak, M.A.; Hindell, M.A. Refining instrument attachment on phocid seals. Mar. Mamm. Sci. 2012, 28, E325–E332. [Google Scholar] [CrossRef]
- Coughlin, C.E.; van Heezik, Y. Weighed down by science: Do collar-mounted devices affect domestic cat behaviour and movement? Wildl. Res. 2015, 41, 606–614. [Google Scholar] [CrossRef]
- Brooks, C.; Bonyongo, C.; Harris, S. Effects of global positioning system collar weight on zebra behavior and location error. J. Wildl. Manag. 2008, 72, 527–534. [Google Scholar] [CrossRef]
- Wilson, R.P.; McMahon, C.R. Measuring devices on wild animals: What constitutes acceptable practice? Front. Ecol. Environ. 2006, 4, 147–154. [Google Scholar] [CrossRef]
- Montané, J.; Marco, I.; Manteca, X.; López, J.; Lavín, S. Delayed acute capture myopathy in three roe deer. J. Vet. Med. Ser. A 2002, 49, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Marco, I.; Mentaberre, G.; Ponjoan, A.; Bota, G.; Mañosa, S.; Lavín, S. Capture myopathy in little bustards after trapping and marking. J. Wildl. Dis. 2006, 42, 889–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breed, D.; Meyer, L.C.R.; Steyl, J.C.A.; Goddard, A.; Burroughs, R.; Kohn, T.A. Conserving wildlife in a changing world: Understanding capture myopathy-a malignant outcome of stress during capture and translocation. Conserv. Physiol. 2020, 7, coz027. [Google Scholar] [CrossRef] [Green Version]
- Chirife, A.D.; Millán, J. Field Immobilization of wood mice (Apodemus sylvaticus) with medetomidine and ketamine and antagonism with atipamezole. J. Wildl. Dis. 2014, 50, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Machin, K.L.; Caulkett, N.A. Evaluation of isoflurance and propofol anesthesia for intra-abdominal transmitter placement in nesting female canvasback ducks. J. Wildl. Dis. 2000, 36, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Millán, J.; Gortázar, C.; Buenestado, F.J.; Marco, I.; Villafuerte, R. Self-injury and capture myopathy in net-captured juvenile red-legged partridge with necklace radiotags. Wildl. Soc. Bull. 2004, 32, 344–350. [Google Scholar] [CrossRef]
- Spotswood, E.N.; Goodman, K.R.; Carlisle, J.; Cormier, R.L.; Humple, D.L.; Rousseau, J.; Guers, S.L.; Barton, G.G. How safe is mist netting? Evaluating the risk of injury and mortality to birds. Methods Ecol. Evol. 2012, 3, 29–38. [Google Scholar] [CrossRef]
- Veldhuizen, L.J.L.; Berentsen, P.B.M.; De Boer, I.J.M.; Van De Vis, J.W.; Bokkers, E.A.M. Fish welfare in capture fisheries: A review of injuries and mortality. Fish. Res. 2018, 204, 41–48. [Google Scholar] [CrossRef]
- Read, J.L.; Pedler, R.D.; Kearney, M.R. Too much hot air? Informing ethical trapping in hot, dry environments. Wildl. Res. 2018, 45, 16–30. [Google Scholar] [CrossRef]
- Perry, D.; Perry, G. Improving interactions between animal rights groups and conservation biologists. Conserv. Biol. 2008, 22, 27–35. [Google Scholar] [CrossRef]
- Waugh, C.A.; Monamy, V. Opposing lethal wildlife research when nonlethal methods exist: Scientific whaling as a case study. J. Fish. Wildl. Manag. 2016, 7, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D. PETA targets early-career wildlife researcher. Science 2017, 357, 1087. [Google Scholar] [CrossRef]
- Bekoff, M. The importance of ethics in conservation biology: Let’s be ethicists, not ostriches. Endanger. Species Update 2002, 19, 23–26. [Google Scholar]
- Vucetich, J.A.; Nelson, M.P. What are 60 warblers worth? Killing in the name of conservation. Oikos 2007, 116, 1267–1278. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Sulikowski, J. Killing for conservation: The need for alternatives to lethal sampling of apex predatory sharks. Endanger. Species Res. 2011, 14, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Minteer, B.A.; Collins, J.P.; Puschendorf, R. Specimen collection: Plan for the future response. Science 2014, 344, 816. [Google Scholar] [CrossRef] [PubMed]
- Cattet, M.; Boulanger, J.; Stenhouse, G.; Powell, R.A.; Reynolds-Hogland, M.L. An evaluation of long-term capture effects in ursids: Implications for wildlife welfare and research. J. Mammal. 2008, 89, 973–990. [Google Scholar] [CrossRef]
- Linhart, P.; Fuchs, R.; Polakova, S.; Slabbekoorn, H. Once bitten twice shy: Long-term behavioural changes caused by trapping experience in willow warblers Phylloscopus trochilus. J. Avian Biol. 2012, 43, 186–192. [Google Scholar] [CrossRef]
- Wilson, R.P.; Sala, J.E.; Gomez-Laich, A.; Ciancio, J.; Quintana, F. Pushed to the limit: Food abundance determines tag-induced harm in penguins. Anim. Welf. 2015, 24, 37–44. [Google Scholar] [CrossRef]
- Zemanova, M.A. More training in animal ethics needed for European biologists. Bioscience 2017, 67, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 1. [Google Scholar]
- Field, K.A.; Paquet, P.C.; Artelle, K.; Proulx, G.; Brook, R.K.; Darimont, C.T. Publication reform to safeguard wildlife from researcher harm. PLoS Biol. 2019, 17, e3000193. [Google Scholar] [CrossRef] [Green Version]
- Zemanova, M.A. Poor implementation of non-invasive sampling in wildlife genetics studies. Rethink. Ecol. 2019, 4, 119–132. [Google Scholar] [CrossRef]
- Robinson, S.; Chapman, K.; Hudson, S.; Sparrow, S.; Spencer-Briggs, D.; Danks, A.; Hill, R.; Everett, D.; Muller, B.; Old, S.; et al. Guidance on Dose Level Selection for Regulatory General Toxicology Studies for Pharmaceuticals; NC3Rs: London, UK, 2009; Available online: https://www.nc3rs.org.uk/sites/default/files/documents/Workshop_reports/Guidance%20on%20dose%20level%20selection%20for%20regulatory%20general%20toxicology%20studies%20for%20pharmaceuticals.pdf (accessed on 10 May 2021).
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.; Jennings, M. Guidance on the Housing and Care of Zebrafish Danio Rerio; Research Animals Department Science Group, RSPCA: Horsham, UK, 2010; Available online: https://science.rspca.org.uk/documents/1494935/9042554/Guidance+on+the+housing+and+care+of+zebrafish.pdf/a4982df2-1499-52bd-d866-9c5706ddda09?t=1552901798437 (accessed on 20 May 2021).
- Zintzsch, A.; Noe, E.; Reissmann, M.; Ullmann, K.; Kramer, S.; Jerchow, B.; Kluge, R.; Gosele, C.; Nickles, H.; Puppe, A.; et al. Guidelines on severity assessment and classification of genetically altered mouse and rat lines. Lab. Anim. 2017, 51, 573–582. [Google Scholar] [CrossRef]
- Díaz, S.; Zafra-Calvo, N.; Purvis, A.; Verburg, P.H.; Obura, D.; Leadley, P.; Chaplin-Kramer, R.; De Meester, L.; Dulloo, E.; Martín-López, B.; et al. Set ambitious goals for biodiversity and sustainability. Science 2020, 370, 411. [Google Scholar] [CrossRef] [PubMed]
- Pinillos, R.G.; Appleby, M.C.; Manteca, X.; Scott-Park, F.; Smith, C.; Velarde, A. One Welfare-a platform for improving human and animal welfare. Vet. Rec. 2016, 179, 412–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horta, O. Animal suffering in nature: The case for intervention. Environ. Ethics 2017, 39, 261–279. [Google Scholar] [CrossRef]
- Cattet, M.R.L. Falling through the cracks: Shortcomings in the collaboration between biologists and veterinarians and their consequences for wildlife. ILAR J. 2013, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.A.H.; Mayer, R.; Osinga, N. Flow simulation along a seal: The impact of an external device. Eur. J. Wildl. Res. 2010, 56, 131–140. [Google Scholar] [CrossRef] [Green Version]

| Year | 2015 | 2016 | 2017 |
|---|---|---|---|
| Animals Used | 38,070 | 71,852 | 78,893 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanova, M.A. New Online Resource on the 3Rs Principles of Animal Research for Wildlife Biologists, Ecologists, and Conservation Managers. Conservation 2021, 1, 106-112. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1020009
Zemanova MA. New Online Resource on the 3Rs Principles of Animal Research for Wildlife Biologists, Ecologists, and Conservation Managers. Conservation. 2021; 1(2):106-112. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1020009
Chicago/Turabian StyleZemanova, Miriam A. 2021. "New Online Resource on the 3Rs Principles of Animal Research for Wildlife Biologists, Ecologists, and Conservation Managers" Conservation 1, no. 2: 106-112. https://0-doi-org.brum.beds.ac.uk/10.3390/conservation1020009






