Overview of Nano-Fiber Mats Fabrication via Electrospinning and Morphology Analysis
Abstract
:1. Review on Electrospinning and Electrostatic Phenomenon
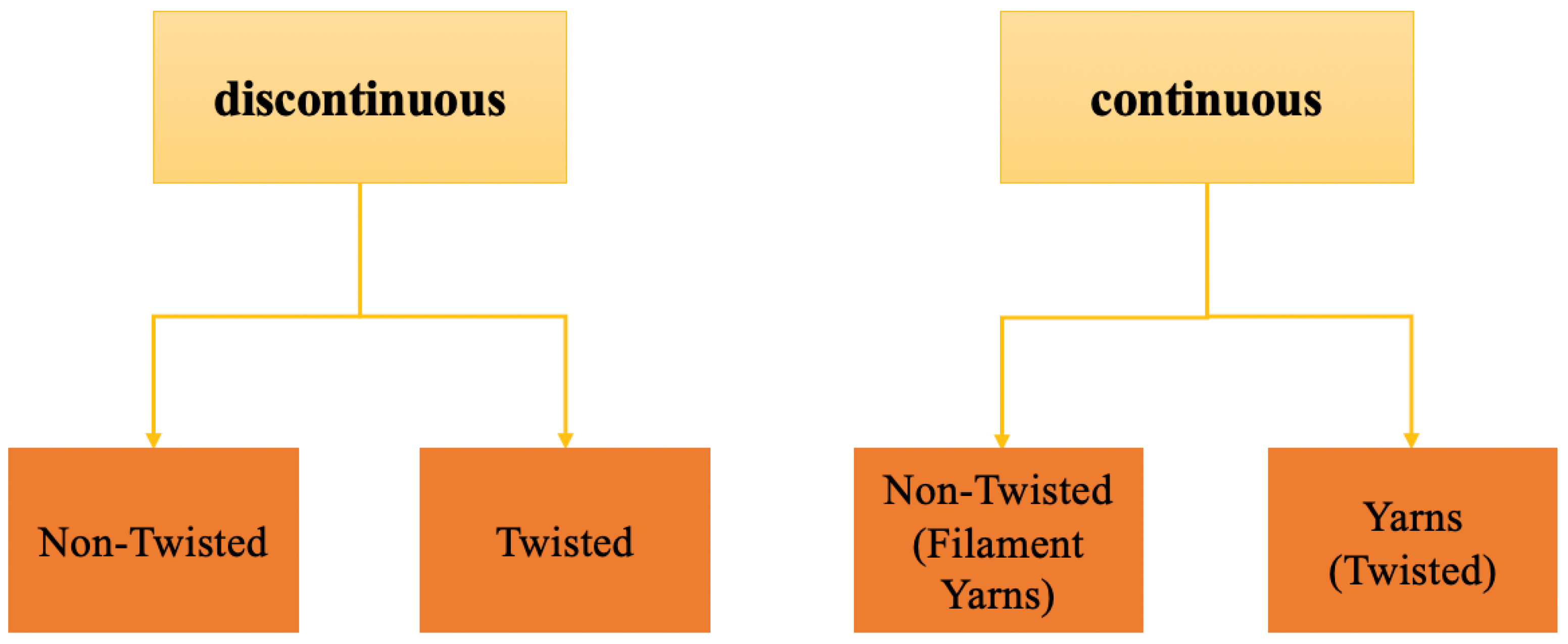
2. Overview of Nano-Fiber Bundles Fabrication Methods and Electrospinning
3. Electrospinning Categories
4. Electrospinning Process and Principles
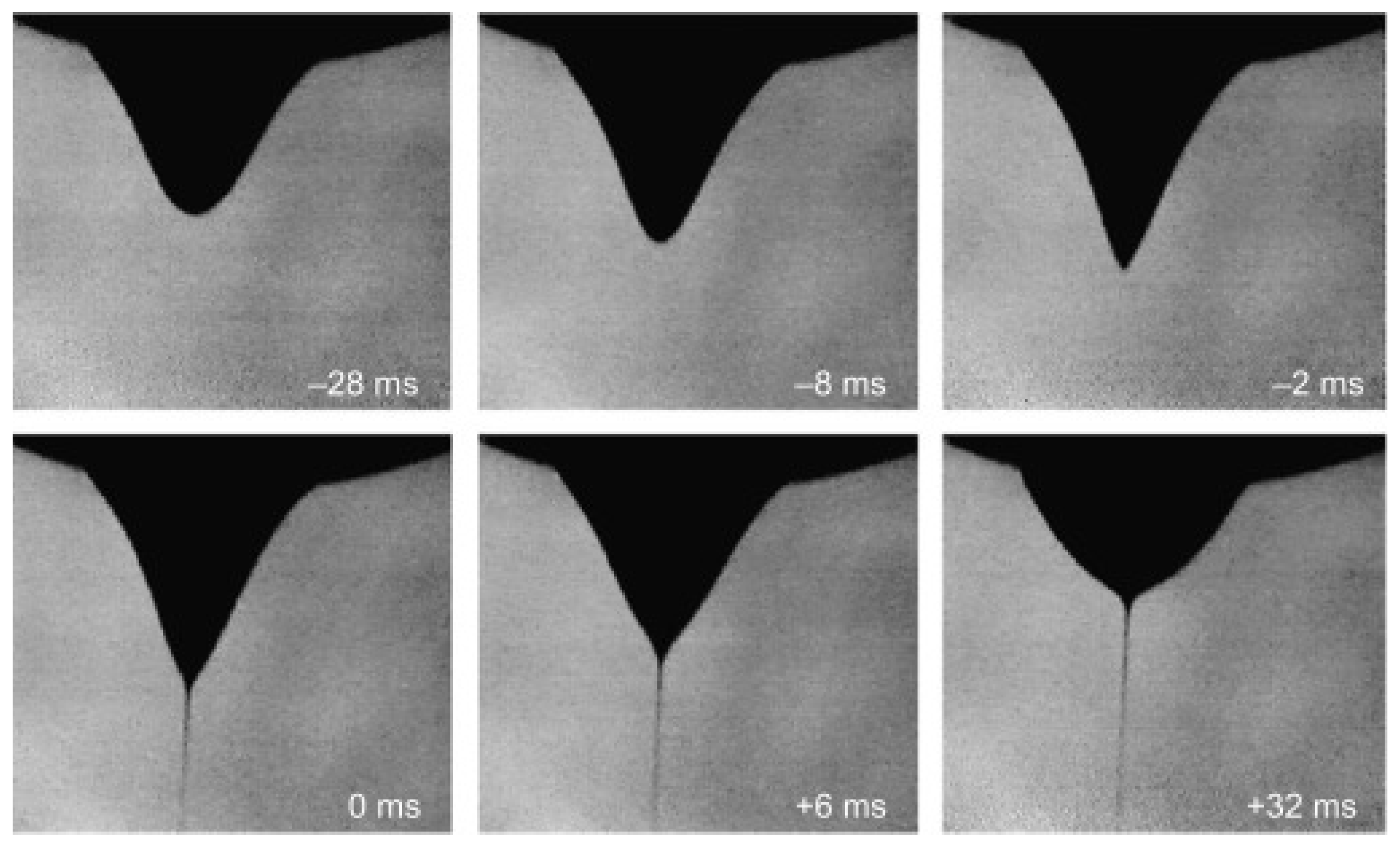
4.1. Taylor Cone Formation
4.2. Whipping and Jet Instability
4.3. Fiber Deposition
5. Solution-Based Electrospinning and Related Effective Parameters
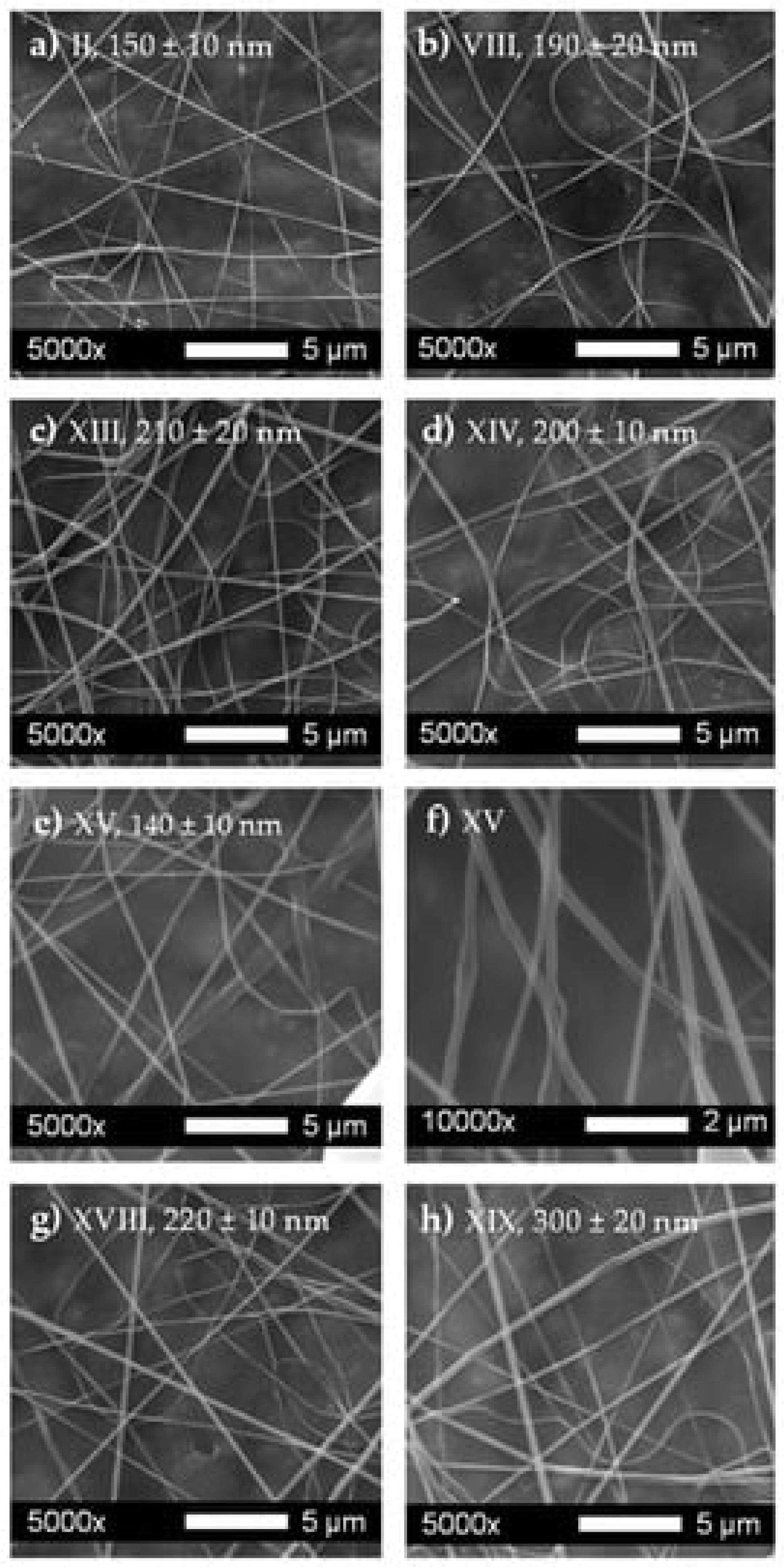
5.1. Concentration and Viscosity
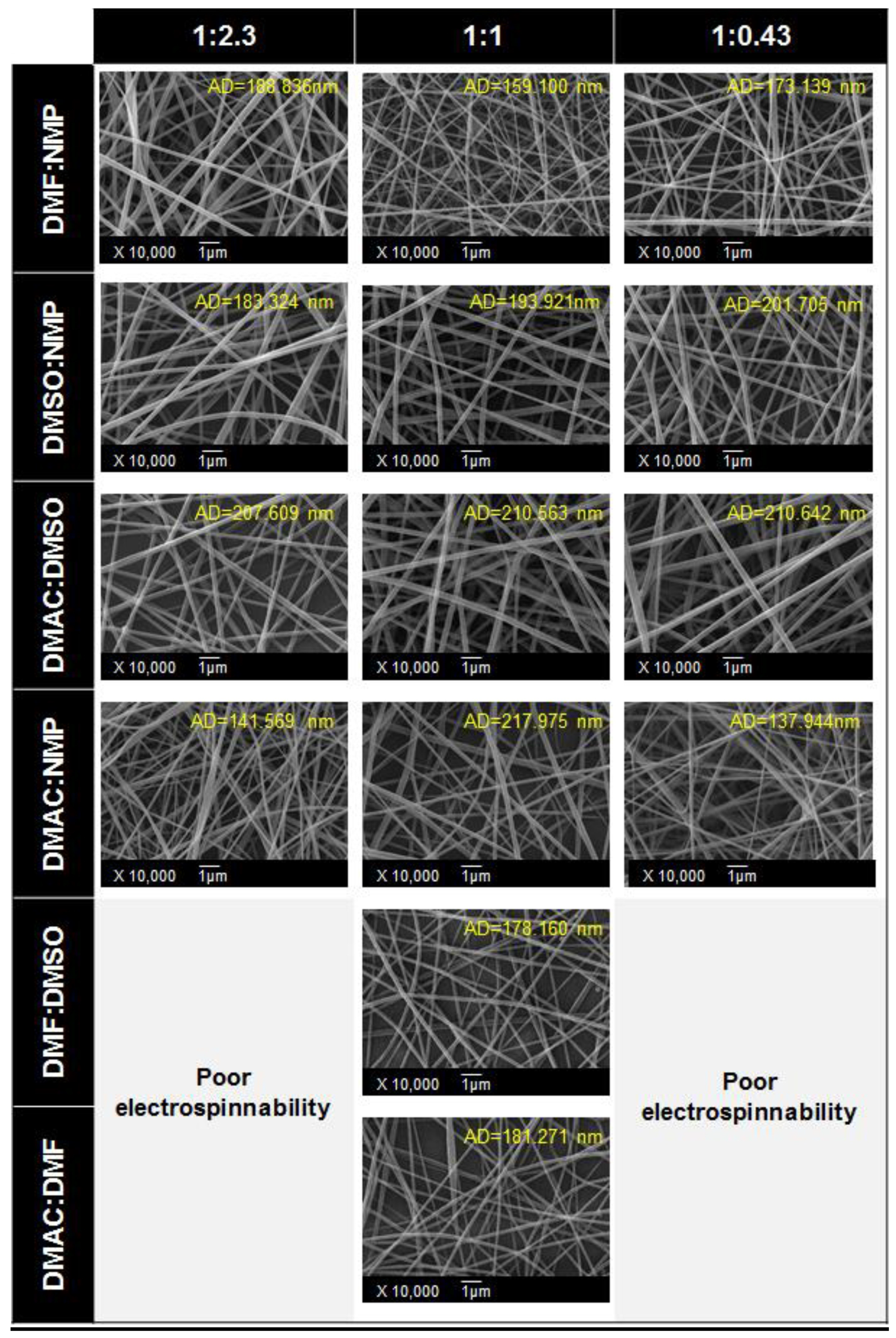
5.2. Solvent
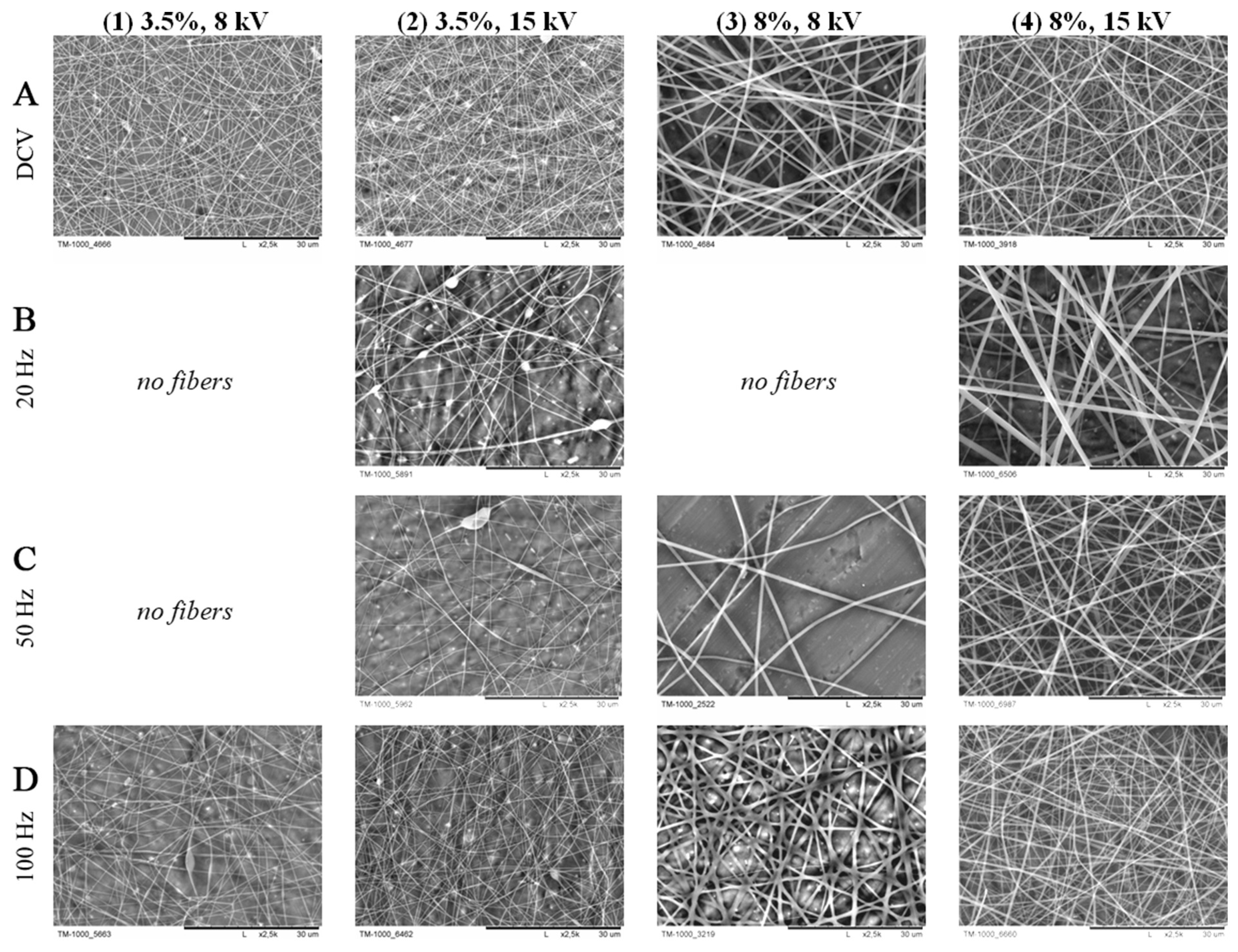
5.3. Voltage and Electric Field
5.4. Flow Rate
5.5. Collecting Distance
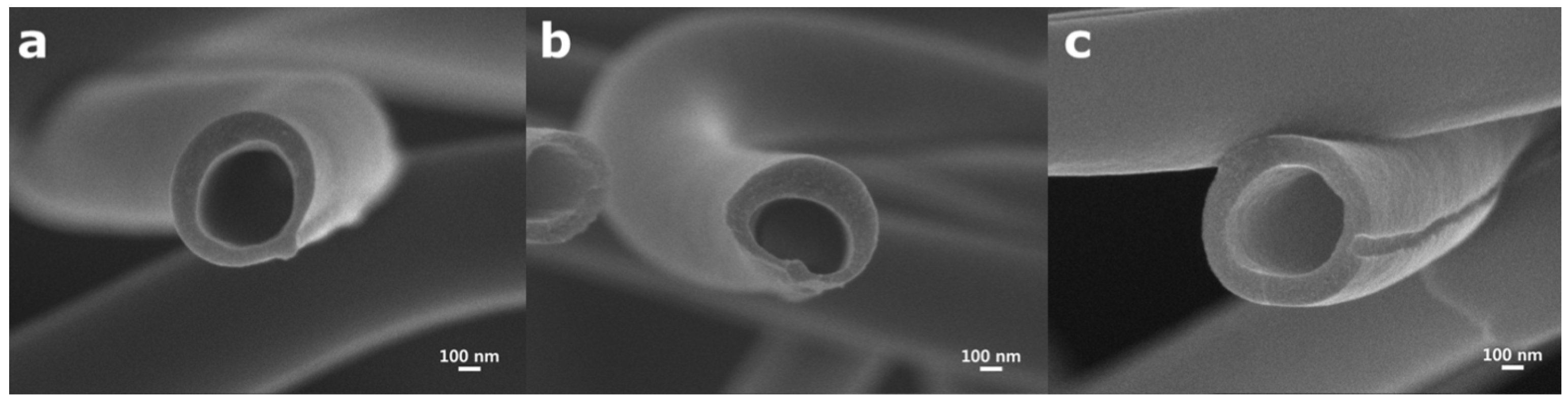
5.6. Nozzle Geometry
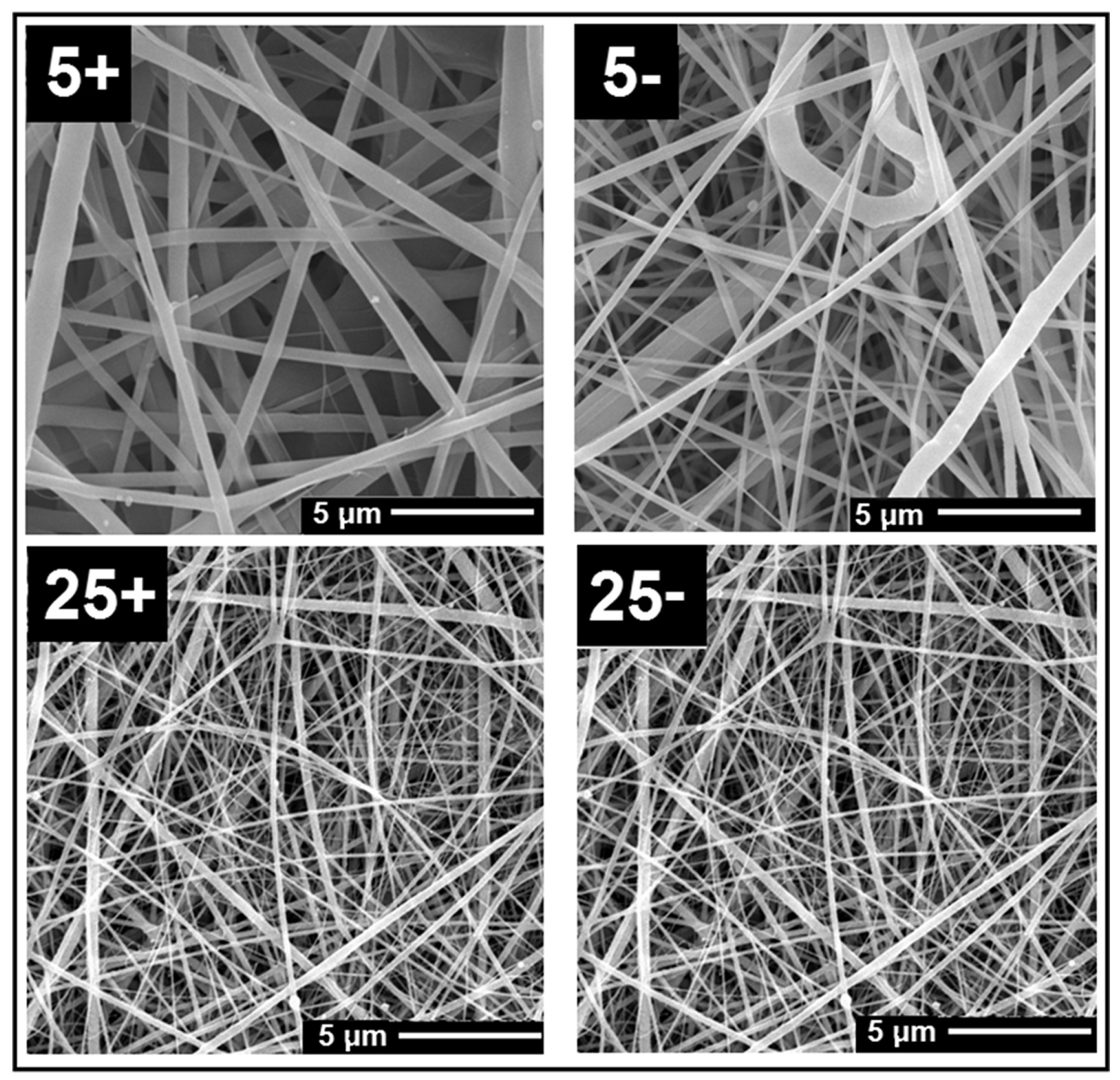
5.7. Polarity
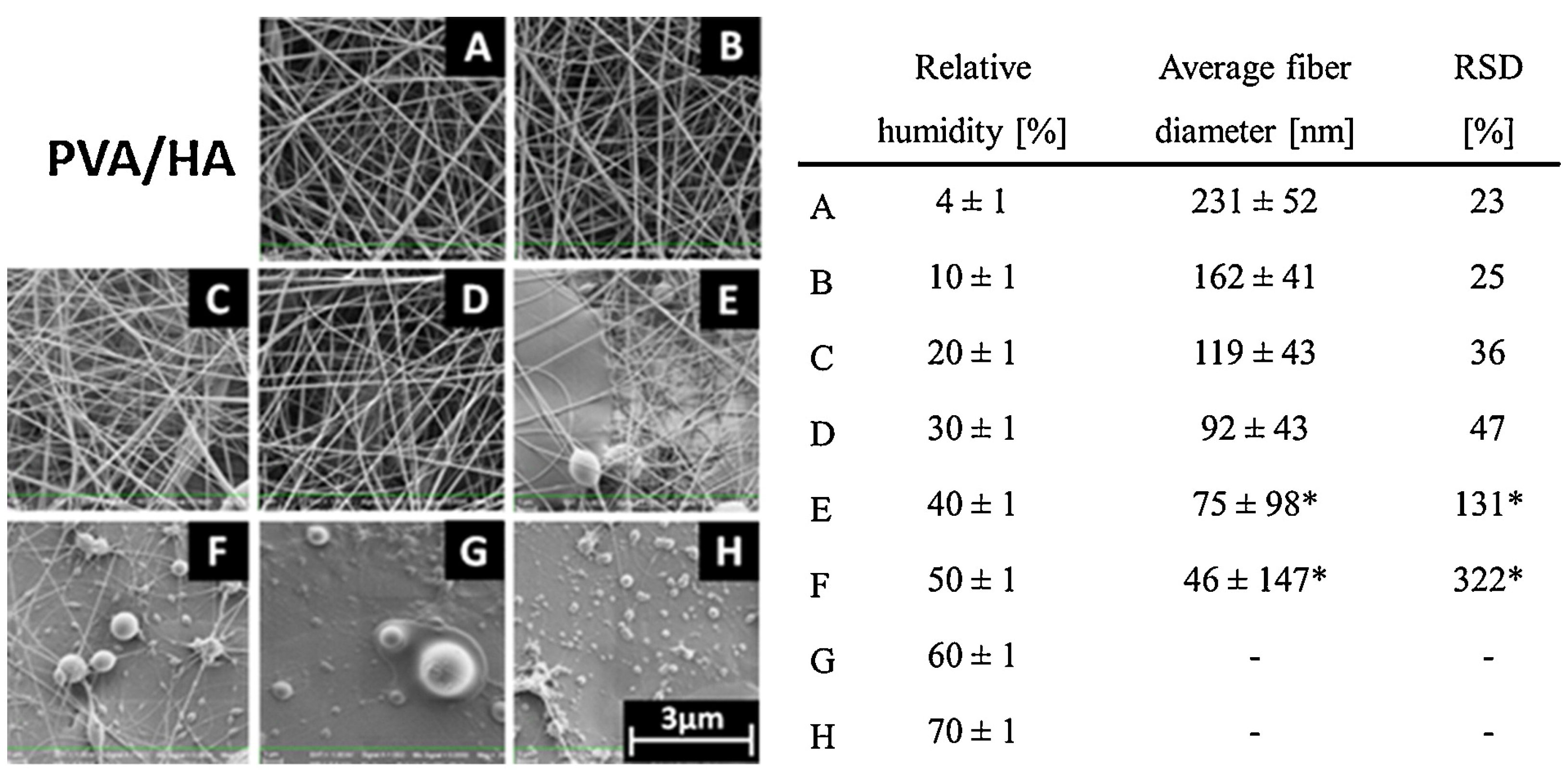
5.8. Humidity
5.9. Temperature
6. Common Polymers in Electrospinning
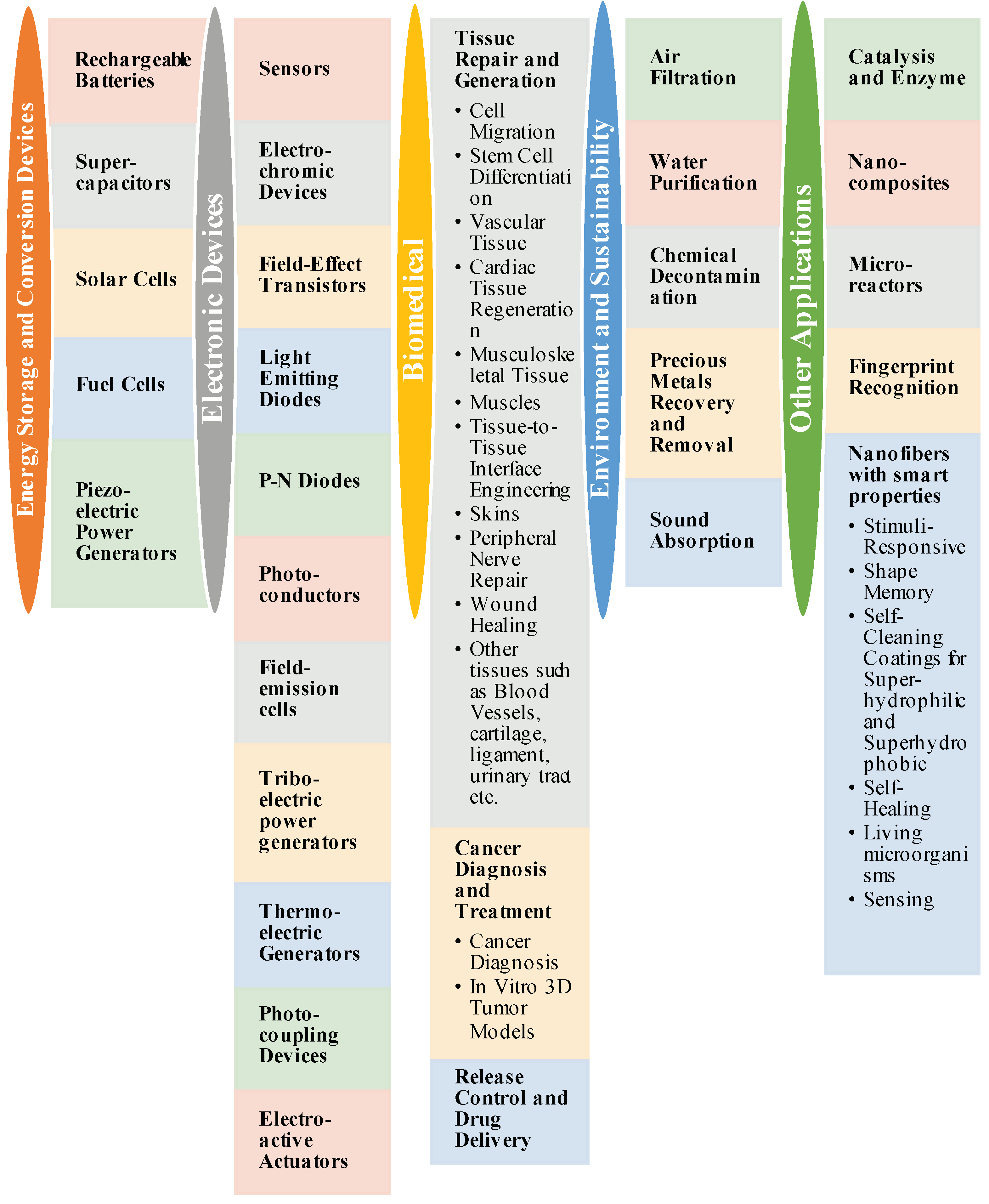
7. Applications of Nano-Fiber Mats via Electrospinning
8. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bora, N.S.; Mazumder, B.; Pathak, M.P.; Joshi, K.; Chattopadhyay, P. Nanotechnology in Preventive and Emergency Healthcare. In Nanotechnology: Therapeutic, Nutraceutical, and Cosmetic Advances; CRC Press: Boca Raton, FL, USA, 2019; p. 221. [Google Scholar]
- Subbiah, T.; Bhat, G.; Tock, R.; Parameswaran, S.; Ramkumar, S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 2 October 1934. [Google Scholar]
- D’Avino, G.; Muccioli, L.; Castet, F.; Poelking, C.; Andrienko, D.; Soos, Z.G.; Cornil, J.; Beljonne, D. Electrostatic phenomena in organic semiconductors: Fundamentals and implications for photovoltaics. J. Phys. Condens. Matter 2016, 28, 433002. [Google Scholar] [CrossRef]
- Xiao, K.; Zhou, Y.; Kong, X.Y.; Xie, G.; Li, P.; Zhang, Z.; Wen, L.; Jiang, L. Electrostatic-charge-and electric-field-induced smart gating for water transportation. ACS Nano 2016, 10, 9703–9709. [Google Scholar] [CrossRef]
- Hughes, J.; Schaub, H. Effects of charged dielectrics on electrostatic force and torque. In International Workshop on Spacecraft Formation Flying; International Astronautical Federation: Boulder, CO, USA, 2017. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Lin, T.; Fang, J. Fundamentals of Electrospinning and Electrospun Nanofibers; DEStech Publications: Lancaster, PA, USA, 2017. [Google Scholar]
- Angammana, C.J.; Jayaram, S.H. Fundamentals of electrospinning and processing technologies. Part. Sci. Technol. 2016, 34, 72–82. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Luo, C.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tuan, R.S. Fabrication and application of nanofibrous scaffolds in tissue engineering. Curr. Protoc. Cell Biol. 2009, 42, 25.2.1–25.2.12. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Yousefzadeh, M. Modeling and simulation of the electrospinning process. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 277–301. [Google Scholar]
- Ahmadian, A. Design and Fabrication of High Capacity Lithium-Ion Batteries Using Electro-Spun Graphene Modified Vanadium Pentoxide Cathodes. Master’s Thesis, Purdue University Graduate School, West Lafayette, IN, USA, 2019. [Google Scholar]
- Shuakat, M.N.; Lin, T. Recent developments in electrospinning of nanofiber yarns. J. Nanosci. Nanotechnol. 2014, 14, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Démuth, B.; Farkas, A.; Pataki, H.; Balogh, A.; Szabó, B.; Borbás, E.; Sóti, P.L.; Vigh, T.; Kiserdei, É.; Farkas, B. Detailed stability investigation of amorphous solid dispersions prepared by single-needle and high speed electrospinning. Int. J. Pharm. 2016, 498, 234–244. [Google Scholar] [CrossRef]
- Susanto, H.; Samsudin, A.; Faz, M.; Rani, M. Impact of post-treatment on the characteristics of electrospun poly (vinyl alcohol)/chitosan nanofibers. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2016; Volume 1725, p. 020087. [Google Scholar]
- Zhou, F.; Gong, R.; Porat, I. Mass production of nanofibre assemblies by electrostatic spinning. Polym. Int. 2009, 58, 331–342. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Lin, T.; Wang, X. Needleless electrospinning of nanofibers with a conical wire coil. Polym. Eng. Sci. 2009, 49, 1582–1586. [Google Scholar] [CrossRef] [Green Version]
- King, M.W.; Gupta, B.S.; Guidoin, R. Biotextiles as Medical Implants; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. I. Stability theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef] [Green Version]
- Cosio, M.S.; Benedetti, S.; Scampicchio, M.; Mannino, S. Electroanalysis in food process control. In Agricultural and Food Electroanalysis; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 421–441. [Google Scholar]
- Guo, H.F.; Xu, B.G. Numerical study of Taylor cone dynamics in electrospinning of nanofibers. In Key Engineering Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2017; Volume 730, pp. 510–515. [Google Scholar]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.; Rivero, J.; Gundabala, V.R.; Perez-Saborid, M.; Fernandez-Nieves, A. Whipping of electrified liquid jets. Proc. Natl. Acad. Sci. USA 2014, 111, 13763–13767. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Jirsak, O.; Sanetrnik, F.; Lukas, D.; Kotek, V.; Martinova, L.; Chaloupek, J. Method of Nanofibres Production from a Polymer Solution Using Electrostatic Spinning and a Device for Carrying Out the Method. U.S. Patent 7,585,437, 8 September 2009. [Google Scholar]
- Luzio, A.; Canesi, E.; Bertarelli, C.; Caironi, M. Electrospun polymer fibers for electronic applications. Materials 2014, 7, 906–947. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M. Controlling nanofiber morphology by the electrospinning process. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–123. [Google Scholar]
- Leach, M.K.; Feng, Z.Q.; Tuck, S.J.; Corey, J.M. Electrospinning fundamentals: Optimizing solution and apparatus parameters. JoVE (J. Vis. Exp.) 2011, 47, e2494. [Google Scholar] [CrossRef] [PubMed]
- Erdem, R.; Usta, I.; Akalin, M.; Atak, O.; Yuksek, M.; Pars, A. The impact of solvent type and mixing ratios of solvents on the properties of polyurethane based electrospun nanofibers. Appl. Surf. Sci. 2015, 334, 227–230. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. Effects of polymer concentration and cationic surfactant on the morphology of electrospun polyacrylonitrile nanofibres. J. Mater. Sci. Technol. 2005, 21, 1–4. [Google Scholar]
- Bercea, M.; Morariu, S.; Ioan, C.; Ioan, S.; Simionescu, B.C. Viscometric study of extremely dilute polyacrylonitrile solutions. Eur. Polym. J. 1999, 35, 2019–2024. [Google Scholar] [CrossRef]
- Moriya, A.; Shen, P.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Reduction of fouling on poly (lactic acid) hollow fiber membranes by blending with poly (lactic acid)–polyethylene glycol–poly (lactic acid) triblock copolymers. J. Membr. Sci. 2012, 415, 712–717. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Vasconcelos, H.; Lobo, J.M.S.; Amaral, H. Delivering miRNA modulators for cancer treatment. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 517–565. [Google Scholar]
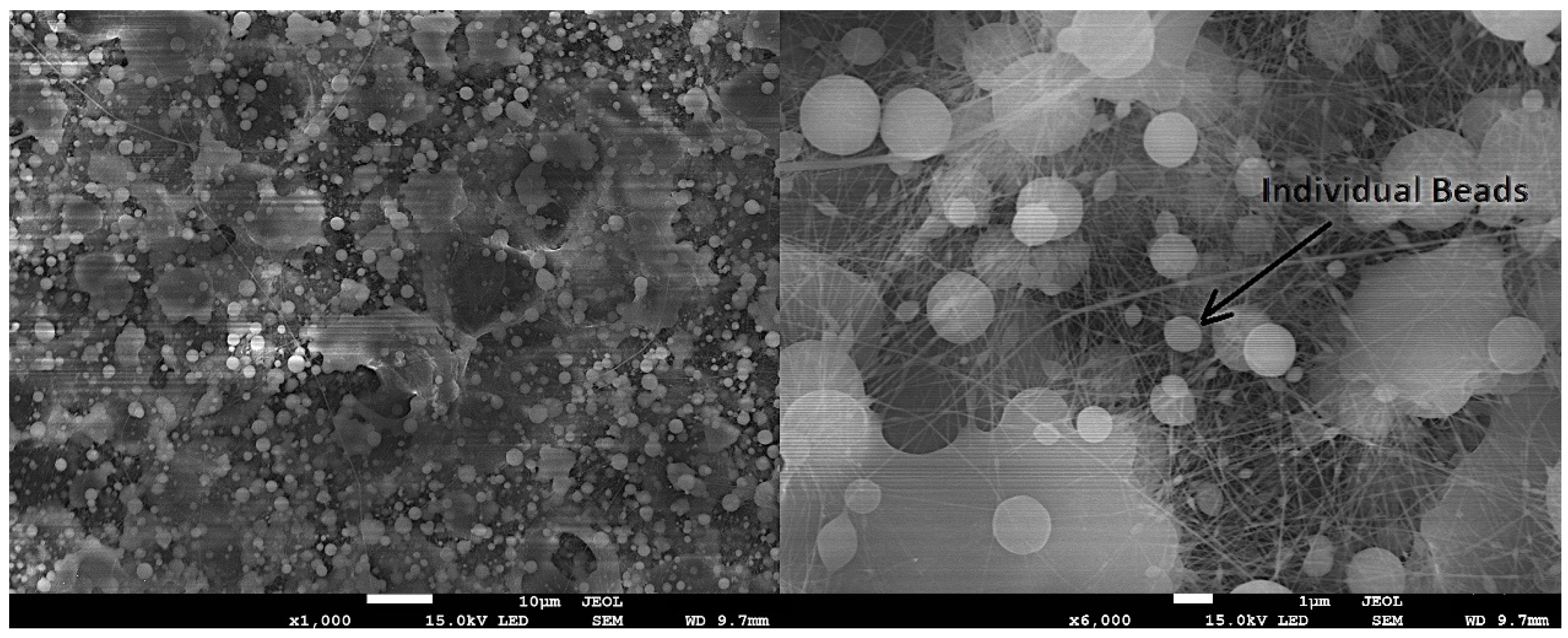
- Hsu, C.M.; Shivkumar, S. Nano-sized beads and porous fiber constructs of poly (ϵ-caprolactone) produced by electrospinning. J. Mater. Sci. 2004, 39, 3003–3013. [Google Scholar] [CrossRef]
- Wu, Y.K.; Wang, L.; Fan, J.; Shou, W.; Zhou, B.M.; Liu, Y. Multi-jet electrospinning with auxiliary electrode: The influence of solution properties. Polymers 2018, 10, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Zeng, Y. Electric field distribution and initial jet motion induced by spinneret configuration for molecular orientation in electrospun fibers. Eur. Polym. J. 2018, 98, 330–336. [Google Scholar] [CrossRef]
- Meechaisue, C.; Dubin, R.; Supaphol, P.; Hoven, V.P.; Kohn, J. Electrospun mat of tyrosine-derived polycarbonate fibers for potential use as tissue scaffolding material. J. Biomater. Sci. Polym. Ed. 2006, 17, 1039–1056. [Google Scholar] [CrossRef]
- Balogh, A.; Cselkó, R.; Démuth, B.; Verreck, G.; Mensch, J.; Marosi, G.; Nagy, Z.K. Alternating current electrospinning for preparation of fibrous drug delivery systems. Int. J. Pharm. 2015, 495, 75–80. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Pálvölgyi, Á.; Domokos, A.; Démuth, B.; Marosi, G.; Nagy, Z.K. Novel alternating current electrospinning of hydroxypropylmethylcellulose acetate succinate (HPMCAS) nanofibers for dissolution enhancement: The importance of solution conductivity. J. Pharm. Sci. 2017, 106, 1634–1643. [Google Scholar] [CrossRef]
- Pokorny, P.; Kostakova, E.; Sanetrnik, F.; Mikes, P.; Chvojka, J.; Kalous, T.; Bilek, M.; Pejchar, K.; Valtera, J.; Lukas, D. Effective AC needleless and collectorless electrospinning for yarn production. Phys. Chem. Chem. Phys. 2014, 16, 26816–26822. [Google Scholar] [CrossRef]
- Kessick, R.; Fenn, J.; Tepper, G. The use of AC potentials in electrospraying and electrospinning processes. Polymer 2004, 45, 2981–2984. [Google Scholar] [CrossRef]
- Mirek, A.; Korycka, P.; Grzeczkowicz, M.; Lewińska, D. Polymer fibers electrospun using pulsed voltage. Mater. Des. 2019, 183, 108106. [Google Scholar] [CrossRef]
- Theron, S.; Zussman, E.; Yarin, A. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Srivastava, R. Electrospinning of patterned and 3D nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 399–447. [Google Scholar]
- Wang, L.; Ryan, A. Introduction to electrospinning. In Electrospinning for Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–33. [Google Scholar]
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-based blends: Processing optimization for their scalable production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubasova, D.; Netravali, A.N. A novel method for electrospinning nanofibrous 3D structures. Fibers 2020, 8, 27. [Google Scholar] [CrossRef]
- Lee, B.S.; Jeon, S.Y.; Park, H.; Lee, G.; Yang, H.S.; Yu, W.R. New electrospinning nozzle to reduce jet instability and its application to manufacture of multi-layered nanofibers. Sci. Rep. 2014, 4, 6758. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Li, H.P.; Yang, J.H.; Wan, J.; Yu, D.G. Influence of working temperature on the formation of electrospun polymer nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Van-Pham, D.T.; Quyen, T.T.B.; Van Toan, P.; Nguyen, C.N.; Ho, M.H.; Thien, D.V.H. Temperature effects on electrospun chitosan nanofibers. Green Process. Synth. 2020, 9, 488–495. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly (methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Wnek, G. Correlations between electrospinnability and physical gelation. Polymer 2005, 46, 8990–9004. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Poudel, B.K.; Doh, K.O.; Byeon, J.H. Green and continuous route to assemble lateral nanodimensional graphitic oxide composites without process interruption. Green Chem. 2018, 20, 2984–2989. [Google Scholar] [CrossRef]
- Akbar, M.; Mortazavi Ashkezari, S.M.J.; Hasani Bidgoli, J.; Ahmadian, A.; Sadeghi, M.J.; Ahmadian, A.; Farahmand, F.; Sarkar, S. Robotic Guide for Brain Biopsy. U.S. Patent 10,555,784, 11 February 2020. [Google Scholar]
- Ahmadian, A.; Hasani Bidgoli, J.; Sadeghi, M.J.; Ahmadian, A.; Farahmand, F.; Sarkar, S. Device for Brain Biopsy. U.S. Patent No. 10,631,947, 28 April 2020. [Google Scholar]
- Ahmadian, A. A Review on Recent Selective Laser Sintering Printing of Medicines; Technical Report; EasyChair: Manchester, UK, 2021. [Google Scholar]
- Korkmaz, S.; Tezel, F.M.; Kariper, I. Synthesis and Characterization of GO/V2O5 Thin Film Supercapacitor. Synth. Met. 2018, 242, 37–48. [Google Scholar] [CrossRef]
- Daemi, N.; Ahmadian, A.; Mirbagheri, A.; Ahmadian, A.; Saberi, H.; Amidi, F.; Alirezaie, J. Planning screw insertion trajectory in lumbar spinal fusion using pre-operative CT images. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3639–3642. [Google Scholar]
- Monyoncho, E.A.; Bissessur, R.; Dahn, D.C.; Trenton, V. Intercalation of poly [oligo (ethylene glycol) oxalate] into vanadium pentoxide xerogel. In Alkali-Ion Batteries; IntechOpen: London, UK, 2016; pp. 93–110. [Google Scholar]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayor, R.; Etienne-Manneville, S. The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 2016, 17, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging roles of electrospun nanofibers in cancer research. Adv. Healthc. Mater. 2018, 7, 1701024. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Rabbi, A.; Bahrambeygi, H.; Shoushtari, A.M.; Nasouri, K. Incorporation of nanofiber layers in non-woven materials for improving their acoustic properties. J. Eng. Fibers Fabr. 2013, 8, 155892501300800412. [Google Scholar]
- Yang, S.; Wang, C.F.; Chen, S. A Release-Induced Response for the Rapid Recognition of Latent Fingerprints and Formation of Inkjet-Printed Patterns. Angew. Chem. Int. Ed. 2011, 50, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacher, P., Jr.; Palacios, M.A. Polymer nanofibre junctions of attolitre volume serve as zeptomole-scale chemical reactors. Nat. Chem. 2009, 1, 80. [Google Scholar] [CrossRef]
- Barbosa, G.N.; Graeff, C.F.O.; Oliveira, H.P. Thermal annealing effects on vanadium pentoxide xerogel films. Eclet. Quim. 2005, 30, 7–15. [Google Scholar] [CrossRef]
- Huang, X.; Bahroloomi, D.; Xiao, X. A multilayer composite separator consisting of non-woven mats and ceramic particles for use in lithium ion batteries. J. Solid State Electrochem. 2014, 18, 133–139. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A modified ceramic-coating separator with high-temperature stability for lithium-ion battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Shafiee, A.; Alidoost, M.; Akbari, A. Flexible Paper-Based Li-ion Batteries: A Review. World J. Eng. Technol. 2021, 9, 285. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, S.; Kong, X.; Xiao, Y.; Fu, L. Synthesis of sulfur encapsulated 3D graphene sponge driven by micro-pump and its application in Li–S battery. J. Mater. 2015, 1, 333–339. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadian, A.; Shafiee, A.; Aliahmad, N.; Agarwal, M. Overview of Nano-Fiber Mats Fabrication via Electrospinning and Morphology Analysis. Textiles 2021, 1, 206-226. https://0-doi-org.brum.beds.ac.uk/10.3390/textiles1020010
Ahmadian A, Shafiee A, Aliahmad N, Agarwal M. Overview of Nano-Fiber Mats Fabrication via Electrospinning and Morphology Analysis. Textiles. 2021; 1(2):206-226. https://0-doi-org.brum.beds.ac.uk/10.3390/textiles1020010
Chicago/Turabian StyleAhmadian, Amirhossein, Abbas Shafiee, Nojan Aliahmad, and Mangilal Agarwal. 2021. "Overview of Nano-Fiber Mats Fabrication via Electrospinning and Morphology Analysis" Textiles 1, no. 2: 206-226. https://0-doi-org.brum.beds.ac.uk/10.3390/textiles1020010





