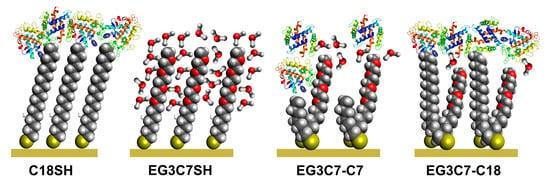
Antifouling Studies of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiol Self-Assembled Monolayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Substrates
2.3. Preparation of SAMs
2.4. Protein Preparation/Exposure
2.5. SPR Procedures
2.6. QCM Measurements
2.7. Ellipsometry Measurements
3. Results and Discussion
3.1. In Situ Analysis of Protein Adsorption Using SPR Spectroscopy
3.2. Ex Situ Analysis of Protein Adsorption Using QCM
3.3. Ellipsometric Thickness Measurements
3.4. Wettability of the SAMs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. A Survey of State-of-the-Art Surface Chemistries to Minimize Fouling from Human and Animal Biofluids. Biomater. Sci. 2015, 3, 1335–1370. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, J.; Kalal, P.; Frutos, A.G.; Jonas, S.J.; Schaeffler, R. Method for Fabricating Supported Bilayer Lipid Membranes on Gold. Langmuir 2000, 16, 7805–7810. [Google Scholar] [CrossRef]
- Lee, H.-H.; Gavutis, M.; Ruželė, Ž.; Valiokas, R.; Liedberg, B. Mixed Self-Assembled Monolayers with Terminal Deuterated Anchors: Characterization and Probing of Model Lipid Membrane Formation. J. Phys. Chem. B 2018, 122, 8201–8210. [Google Scholar] [CrossRef]
- Herrwerth, S.; Rosendahl, T.; Feng, C.; Fick, J.; Eck, W.; Himmelhaus, M.; Dahint, R.; Grunze, M. Covalent Coupling of Antibodies to Self-Assembled Monolayers of Carboxy-Functionalized Poly(ethylene glycol): Protein Resistance and Specific Binding of Biomolecules. Langmuir 2003, 19, 1880–1887. [Google Scholar] [CrossRef]
- Castner, D.G.; Ratner, B.D. Biomedical Surface Science: Foundations to Frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Ko, B.S.; Babcock, B.; Jennings, G.K.; Tilden, S.G.; Peterson, R.R.; Cliffel, D.; Greenbaum, E. Effect of Surface Composition on the Adsorption of Photosystem I onto Alkanethiolate Self-Assembled Monolayers on Gold. Langmuir 2004, 20, 4033–4038. [Google Scholar] [CrossRef]
- Sofia, S.J.; Premnath, V.; Merrill, E.W. Poly(ethylene oxide) Grafted to Silicon Surfaces: Grafting Density and Protein Adsorption. Macromolecules 1998, 31, 5059–5070. [Google Scholar] [CrossRef]
- Clare, T.L.; Clare, B.H.; Nichols, B.M.; Abbott, N.L.; Hamers, R.J. Functional Monolayers for Improved Resistance to Protein Adsorption: Oligo(ethylene glycol)-Modified Silicon and Diamond Surfaces. Langmuir 2005, 21, 6344–6355. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Self-Assembled Organic Monolayers: Model Systems for Studying Adsorption of Proteins at Surfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prime, K.L.; Whitesides, G.M. Adsorption of Proteins onto Surfaces Containing End-Attached Oligo(ethylene oxide): A Model System Using Self-Assembled Monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Luk, Y.-Y.; Tingey, M.L.; Hall, D.J.; Israel, B.A.; Murphy, C.J.; Bertics, P.J.; Abbott, N.L. Using Liquid Crystals to Amplify Protein−Receptor Interactions: Design of Surfaces with Nanometer-Scale Topography That Present Histidine-Tagged Protein Receptors. Langmuir 2003, 19, 1671–1680. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability to Resist Protein Adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Feldman, K.; Hähner, G.; Spencer, N.D.; Harder, P.; Grunze, M. Probing Resistance to Protein Adsorption of Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers by Scanning Force Microscopy. J. Am. Chem. Soc. 1999, 121, 10134–10141. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Zheng, J.; Ratner, B.D.; Jiang, S. Protein Adsorption on Oligo(ethylene glycol)-Terminated Alkanethiolate Self-Assembled Monolayers: The Molecular Basis for Nonfouling Behavior. J. Phys. Chem. B 2005, 109, 2934–2941. [Google Scholar] [CrossRef]
- Sun, K.; Song, L.; Xie, Y.; Liu, D.; Wang, D.; Wang, Z.; Ma, W.; Zhu, J.; Jiang, X. Using Self-Polymerized Dopamine to Modify the Antifouling Property of Oligo(ethylene glycol) Self-Assembled Monolayers and Its Application in Cell Patterning. Langmuir 2011, 27, 5709–5712. [Google Scholar] [CrossRef] [PubMed]
- Krakert, S.; Ballav, N.; Zharnikov, M.; Terfort, A. Adjustment of the Bioresistivity by Electron Irradiation: Self-Assembled Monolayers of Oligo(ethyleneglycol)-Terminated Alkanethiols with Embedded Cleavable Group. Phys. Chem. Chem. Phys. 2010, 12, 507–515. [Google Scholar] [CrossRef]
- Zorn, S.; Skoda, M.W.A.; Gerlach, A.; Jacobs, R.M.J.; Schreiber, F. On the Stability of Oligo(ethylene glycol) (C11EG6OMe) SAMs on Gold: Behavior at Elevated Temperature in Contact with Water. Langmuir 2011, 27, 2237–2243. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Chen, S.; Jiang, S. Molecular Simulation Study of Water Interactions with Oligo (ethylene glycol)-Terminated Alkanethiol Self-Assembled Monolayers. Langmuir 2004, 20, 8931–8938. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—Surface Interactions in the Presence of Polyethylene Oxide: I. Simplified Theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Jeon, S.I.; Andrade, J.D. Protein—Surface Interactions in the Presence of Polyethylene Oxide: II. Effect of Protein Size. J. Colloid Interface Sci. 1991, 142, 159–166. [Google Scholar] [CrossRef]
- McPherson, T.; Kidane, A.; Szleifer, I.; Park, K. Prevention of Protein Adsorption by Tethered Poly(ethylene oxide) Layers: Experiments and Single-Chain Mean-Field Analysis. Langmuir 1998, 14, 176–186. [Google Scholar] [CrossRef]
- Pertsin, A.J.; Grunze, M. Computer Simulation of Water near the Surface of Oligo(ethylene glycol)-Terminated Alkanethiol Self-Assembled Monolayers. Langmuir 2000, 16, 8829–8841. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors That Determine the Protein Resistance of Oligoether Self-Assembled Monolayers—Internal Hydrophilicity, Terminal Hydrophilicity, and Lateral Packing Density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Hayashi, T.; Tanaka, Y.; Koide, Y.; Tanaka, M.; Hara, M. Mechanism Underlying Bioinertness of Self-Assembled Monolayers of Oligo(ethyleneglycol)-Terminated Alkanethiols on Gold: Protein Adsorption, Platelet Adhesion, and Surface Forces. Phys. Chem. Chem. Phys. 2012, 14, 10196–10206. [Google Scholar] [CrossRef]
- Sekine, T.; Tanaka, Y.; Sato, C.; Tanaka, M.; Hayashi, T. Evaluation of Factors to Determine Platelet Compatibility by Using Self-Assembled Monolayers with a Chemical Gradient. Langmuir 2015, 31, 7100–7105. [Google Scholar] [CrossRef]
- Sayin, M.; Nefedov, A.; Zharnikov, M. Interaction of Water with Oligo(ethylene glycol) Terminated Monolayers: Wetting versus Hydration. Phys. Chem. Chem. Phys. 2020, 22, 8088–8095. [Google Scholar] [CrossRef]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Chinwangso, P.; Jamison, A.C.; Lee, T.R. Multidentate Adsorbates for Self-Assembled Monolayer Films. Acc. Chem. Res. 2011, 44, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Shon, Y.-S.; Lee, T.R. Chelating Self-Assembled Monolayers on Gold Generated from Spiroalkanedithiols. Langmuir 1999, 15, 1136–1140. [Google Scholar] [CrossRef]
- Shon, Y.-S.; Lee, T.R. Desorption and Exchange of Self-Assembled Monolayers (SAMs) on Gold Generated from Chelating Alkanedithiols. J. Phys. Chem. B 2000, 104, 8192–8200. [Google Scholar] [CrossRef]
- Shon, Y.-S.; Lee, S.; Perry, S.S.; Lee, T.R. The Adsorption of Unsymmetrical Spiroalkanedithiols onto Gold Affords Multi-Component Interfaces That Are Homogeneously Mixed at the Molecular Level. J. Am. Chem. Soc. 2000, 122, 1278–1281. [Google Scholar] [CrossRef]
- Shon, Y.-S.; Lee, S.; Colorado, R.; Perry, S.S.; Lee, T.R. Spiroalkanedithiol-Based SAMs Reveal Unique Insight into the Wettabilities and Frictional Properties of Organic Thin Films. J. Am. Chem. Soc. 2000, 122, 7556–7563. [Google Scholar] [CrossRef]
- Hoang, J.; Park, C.S.; Marquez, M.D.; Gunaratne, P.H.; Lee, T.R. DNA Binding on Self-Assembled Monolayers Terminated with Mixtures of Ammonium and Trimethylammonium Groups: Toward a Gene-Delivery Platform. ACS Appl. Nano Mater. 2020, 3, 6621–6628. [Google Scholar] [CrossRef]
- Sakunkaewkasem, S.; Gonzalez, M.A.; Marquez, M.D.; Lee, T.R. Olefin-Bridged Bidentate Adsorbates for Generating Self-Assembled Monolayers on Gold. Langmuir 2020, 36, 10699–10707. [Google Scholar] [CrossRef]
- Chinwangso, P.; Lee, H.J.; Lee, T.R. Self-Assembled Monolayers Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. Langmuir 2015, 31, 13341–13349. [Google Scholar] [CrossRef]
- Chinwangso, P.; Lee, H.J.; Jamison, A.C.; Marquez, M.D.; Park, C.S.; Lee, T.R. Structure, Wettability, and Thermal Stability of Organic Thin-Films on Gold Generated from the Molecular Self-Assembly of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiols. Langmuir 2017, 33, 1751–1762. [Google Scholar] [CrossRef]
- Chinwangso, P.; St. Hill, L.R.; Marquez, M.D.; Lee, T.R. Unsymmetrical Spiroalkanedithiols Having Mixed Fluorinated and Alkyl Tailgroups of Varying Length: Film Structure and Interfacial Properties. Molecules 2018, 23, 2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St. Hill, L.R.; Craft, J.W.; Chinwangso, P.; Tran, H.-V.; Marquez, M.D.; Lee, T.R. Antifouling Coatings Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. ACS Appl. Bio Mater. 2021, 4, 1563–1572. [Google Scholar] [CrossRef]
- Smith, R.K.; Reed, S.M.; Lewis, P.A.; Monnell, J.D.; Clegg, R.S.; Kelly, K.F.; Bumm, L.A.; Hutchison, J.E.; Weiss, P.S. Phase Separation within a Binary Self-Assembled Monolayer on Au{111} Driven by an Amide-Containing Alkanethiol. J. Phys. Chem. B 2001, 105, 1119–1122. [Google Scholar] [CrossRef]
- Jackson, A.M.; Myerson, J.W.; Stellacci, F. Spontaneous Assembly of Subnanometre-Ordered Domains in the Ligand Shell of Monolayer-Protected Nanoparticles. Nat. Mater. 2004, 3, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Yaliraki, S.N.; Longo, G.; Gale, E.; Szleifer, I.; Ratner, M.A. Stability and Phase Separation in Mixed Self-Assembled Monolayers. J. Chem. Phys. 2006, 125, 074708. [Google Scholar] [CrossRef] [PubMed]
- Fetisov, E.O.; Siepmann, J.I. Structure and Phase Behavior of Mixed Self-Assembled Alkanethiolate Monolayers on Gold Nanoparticles: A Monte Carlo Study. J. Phys. Chem. B 2016, 120, 1972–1978. [Google Scholar] [CrossRef]
- Gao, J.; Lin, H.; Qin, X.; Zhang, X.; Ding, H.; Wang, Y.; Rokni Fard, M.; Kaya, D.; Zhu, G.; Li, Q.; et al. Probing Phase Evolutions of Au-Methyl-Propyl-Thiolate Self-Assembled Monolayers on Au(111) at the Molecular Level. J. Phys. Chem. B 2018, 122, 6666–6672. [Google Scholar] [CrossRef]
- Akimov, Y.; Pam, M.E.; Sun, S. Kretschmann-Raether Configuration: Revision of the Theory of Resonant Interaction. Phys. Rev. B 2017, 96, 155433. [Google Scholar] [CrossRef]
- Buttry, D.A.; Ward, M.D. Measurement of Interfacial Processes at Electrode Surfaces with the Electrochemical Quartz Crystal Microbalance. Chem. Rev. 1992, 92, 1355–1379. [Google Scholar] [CrossRef]
- Tsai, D.-H.; DelRio, F.W.; Keene, A.M.; Tyner, K.M.; MacCuspie, R.I.; Cho, T.J.; Zachariah, M.R.; Hackley, V.A. Adsorption and Conformation of Serum Albumin Protein on Gold Nanoparticles Investigated Using Dimensional Measurements and in Situ Spectroscopic Methods. Langmuir 2011, 27, 2464–2477. [Google Scholar] [CrossRef]
- Pietrocola, G.; Visai, L.; Valtulina, V.; Vignati, E.; Rindi, S.; Arciola, C.R.; Piazza, R.; Speziale, P. Multiple Interactions of FbsA, a Surface Protein from Streptococcus Agalactiae, with Fibrinogen: Affinity, Stoichiometry, and Structural Characterization. Biochemistry 2006, 45, 12840–12852. [Google Scholar] [CrossRef]
- Furlan, M. Sticky and Promiscuous Plasma Proteins Maintain the Equilibrium between Bleeding and Thrombosis. Swiss Med. Wkly. 2002, 132, 181–189. [Google Scholar]
- Colvin, J.R. The Size and Shape of Lysozyme. Can. J. Chem. 1952, 30, 831–834. [Google Scholar] [CrossRef]
- He, H.; Ye, J.; Liu, E.; Liang, Q.; Liu, Q.; Yang, V.C. Low Molecular Weight Protamine (LMWP): A Nontoxic Protamine Substitute and an Effective Cell-Penetrating Peptide. J. Control. Release 2014, 193, 63–73. [Google Scholar] [CrossRef]
- Marucco, A.; Catalano, F.; Fenoglio, I.; Turci, F.; Martra, G.; Fubini, B. Possible Chemical Source of Discrepancy between in Vitro and in Vivo Tests in Nanotoxicology Caused by Strong Adsorption of Buffer Components. Chem. Res. Toxicol. 2015, 28, 87–91. [Google Scholar] [CrossRef]
- Parkes, M.; Myant, C.; Cann, P.M.; Wong, J.S.S. The Effect of Buffer Solution Choice on Protein Adsorption and Lubrication. Tribol. Int. 2014, 72, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiar, R. Surface Plasmon Resonance Spectroscopy: A Versatile Technique in a Biochemist’s Toolbox. J. Chem. Educ. 2013, 90, 203–209. [Google Scholar] [CrossRef]
- Höök, F.; Rodahl, M.; Kasemo, B.; Brzezinski, P. Structural Changes in Hemoglobin during Adsorption to Solid Surfaces: Effects of PH, Ionic Strength, and Ligand Binding. Proc. Natl. Acad. Sci. USA 1998, 95, 12271–12276. [Google Scholar] [CrossRef] [Green Version]
- Rickert, J.; Brecht, A.; Göpel, W. Quartz Crystal Microbalances for Quantitative Biosensing and Characterizing Protein Multilayers. Biosens. Bioelectron. 1997, 12, 567–575. [Google Scholar] [CrossRef]
- Levi, M.D.; Daikhin, L.; Aurbach, D.; Presser, V. Quartz Crystal Microbalance with Dissipation Monitoring (EQCM-D) for in-Situ Studies of Electrodes for Supercapacitors and Batteries: A Mini-Review. Electrochem. Commun. 2016, 67, 16–21. [Google Scholar] [CrossRef]






| Protein | Protamine | Lysozyme | BSA | Fibrinogen |
|---|---|---|---|---|
| Molecular weight | 4 KDa | 14 KDa | 55 KDa | 340 KDa |
| Size | 5 Å c | 18 Å c | 140 × 40 × 40 Å | 450 × 90 Å |
| Shape | Spherical | Stubby prolate ellipsoid | Prolate ellipsoid a | Cylindrical b |
| pI | 12.1 | 11.1 | 4.8 | 5.7 |
| Application | Insulin | Cell | Blood | Muscle/Tissue |
| SAM | ∆ RU | |||
|---|---|---|---|---|
| Protamine | Lysozyme | BSA | Fibrinogen | |
| C18SH | 599 ± 3 | 1029 ± 42 | 899 ± 63 | 2285 ± 222 |
| EG3C7SH | 173 ± 11 | 108 ± 20 | 195 ± 53 | 201 ± 46 |
| EG3C7-C7 | 368 ± 87 | 1163 ± 77 | 1121 ± 145 | 2194 ± 122 |
| EG3C7-C18 | 936 ± 48 | 1524 ± 80 | 1051 ± 96 | 2295 ± 24 |
| SAM | Protein Loading (ng/cm2) | |||
|---|---|---|---|---|
| Protamine | Lysozyme | BSA | Fibrinogen | |
| C18SH | 329 ± 34 | 409 ± 67 | 493 ± 76 | 854 ± 80 |
| EG3C7SH | 218 ± 94 | 264 ± 12 | 223 ± 75 | 119 ± 30 |
| EG3C7-C7 | 308 ± 40 | 314 ± 62 | 621 ± 72 | 471 ± 52 |
| EG3C7-C18 | 398 ± 45 | 442 ± 76 | 644 ± 99 | 943 ± 83 |
| SAM | ∆ Thickness (Å) | |||
|---|---|---|---|---|
| Protamine | Lysozyme | BSA | Fibrinogen | |
| C18SH | 11 ± 5 | 20 ± 2 | 13 ± 8 | 43 ± 2 |
| EG3C7SH | 2 ± 2 | 2 ± 1 | 2 ± 3 | 3 ± 1 |
| EG3C7-C7 | 3 ± 3 | 19 ± 2 | 8 ± 2 | 27 ± 1 |
| EG3C7-C18 | 9 ± 4 | 20 ± 7 | 16 ± 4 | 39 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
St. Hill, L.R.; Tran, H.-V.; Chinwangso, P.; Lee, H.J.; Marquez, M.D.; Craft, J.W., Jr.; Lee, T.R. Antifouling Studies of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiol Self-Assembled Monolayers. Micro 2021, 1, 151-163. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010012
St. Hill LR, Tran H-V, Chinwangso P, Lee HJ, Marquez MD, Craft JW Jr., Lee TR. Antifouling Studies of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiol Self-Assembled Monolayers. Micro. 2021; 1(1):151-163. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010012
Chicago/Turabian StyleSt. Hill, Lydia R., Hung-Vu Tran, Pawilai Chinwangso, Han Ju Lee, Maria D. Marquez, John W. Craft, Jr., and T. Randall Lee. 2021. "Antifouling Studies of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiol Self-Assembled Monolayers" Micro 1, no. 1: 151-163. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010012