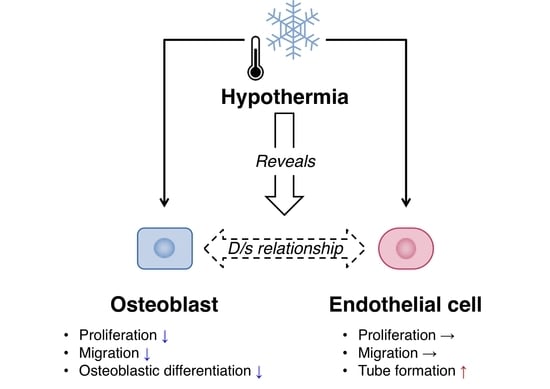
Moderate Hypothermia Has the Potential to Reveal the Dominant/Submissive Relationship in a Co-Culture System Consisting of Osteoblasts and Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Proliferation
2.3. Cell Migration
2.4. Osteoblastic Differentiation
2.5. Endothelial Cell Tube Formation
2.6. Co-Culture of Osteoblasts and Endothelial Cells
2.7. Quantitative Measurement of Alkaline Phosphatase Activity
2.8. Immunofluorescence Analysis of CD31 Expression
2.9. Histochemical Staining for ALP Activity and Immunocytochemical Staining for CD31 Expression
2.10. Statistical Analysis
3. Results
3.1. Effect of Hypothermia on the Osteoblast and the Endothelial Cell Monocultures
3.2. Effects of Hypothermia on the Co-Culture of Osteoblasts and Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cameron, J.A.; Milner, D.J.; Lee, J.S.; Cheng, J.; Fang, N.X.; Jasiuk, I.M. Employing the biology of successful fracture repair to heal critical size bone defects. Curr. Top. Microbiol. Immunol. 2013, 367, 113–132. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.-M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of Bone grafting: Bone grafts and tissue engineering strategies for vascularized bone regeneration. Clin. Rev. Bone Miner. Metab. 2015, 13, 232–244. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized bone tissue engineering: Approaches for potential improvement. Tissue Eng. Part B Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Knothe Tate, M.L. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012, 1, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Gruneboom, A.; Hawwari, I.; Weidner, D.; Culemann, S.; Muller, S.; Henneberg, S.; Brenzel, A.; Merz, S.; Bornemann, L.; Zec, K.; et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 2019, 1, 236–250. [Google Scholar] [CrossRef] [Green Version]
- Herzog, D.P.; Dohle, E.; Bischoff, I.; Kirkpatrick, C.J. Cell communication in a coculture system consisting of outgrowth endothelial cells and primary osteoblasts. Biomed. Res. Int. 2014, 2014, 320123. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Hofmann, A.; Kirkpatrick, C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007, 13, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Bourget, C.; Remy-Zolgadri, M.; Bareille, R.; Fernandez, P.; Conrad, V.; Amedee-Vilamitjana, J. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell. Physiol. Biochem. 2004, 14, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Fuchs, S.; Kolbe, M.; Hofmann, A.; Schmidt, H.; Kirkpatrick, C.J. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng. Part A 2010, 16, 1235–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villars, F.; Guillotin, B.; Amedee, T.; Dutoya, S.; Bordenave, L.; Bareille, R.; Amedee, J. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am. J. Physiol.-Cell Physiol. 2002, 282, C775–C785. [Google Scholar] [CrossRef] [Green Version]
- Simunovic, F.; Winninger, O.; Strassburg, S.; Koch, H.G.; Finkenzeller, G.; Stark, G.B.; Lampert, F.M. Increased differentiation and production of extracellular matrix components of primary human osteoblasts after cocultivation with endothelial cells: A quantitative proteomics approach. J. Cell. Biochem. 2019, 120, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schedle, A.; Matejka, M.; Rausch-Fan, X.; Andrukhov, O. The proliferation and differentiation of osteoblasts in co-culture with human umbilical vein endothelial cells: An improved analysis using fluorescence-activated cell sorting. Cell. Mol. Biol. Lett. 2010, 15, 517–529. [Google Scholar] [CrossRef]
- Ma, B.; Li, M.; Fuchs, S.; Bischoff, I.; Hofmann, A.; Unger, R.E.; Kirkpatrick, C.J. Short-term hypoxia promotes vascularization in co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. J. Biomed. Mater. Res. A 2019. [Google Scholar] [CrossRef]
- Barron, M.J.; Goldman, J.; Tsai, C.J.; Donahue, S.W. Perfusion flow enhances osteogenic gene expression and the infiltration of osteoblasts and endothelial cells into three-dimensional calcium phosphate scaffolds. Int. J. Biomater. 2012, 2012, 915620. [Google Scholar] [CrossRef] [Green Version]
- Shui, C.X.; Scutt, A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J. Bone Miner. Res. 2001, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Utting, J.C.; Key, M.L.; Orriss, I.R.; Taylor, S.E.; Whatling, P.; Arnett, T.R. Hypothermia inhibits osteoblast differentiation and bone formation but stimulates osteoclastogenesis. Exp. Cell Res. 2012, 318, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Guo, S.; Zhang, T.; Li, H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett. 2009, 583, 2500–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, K.; Hinata, Y.; Kagawa, Y.; Iwasaki, K.; Tsuneda, S.; Shimizu, T.; Umezu, M. Low-temperature culturing improves survival rate of tissue-engineered cardiac cell sheets. Biochem. Biophys. Rep. 2018, 14, 89–97. [Google Scholar] [CrossRef]
- Li, M.; Fuchs, S.; Bose, T.; Schmidt, H.; Hofmann, A.; Tonak, M.; Unger, R.; Kirkpatrick, C.J. Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue Eng. Part C Methods 2014, 20, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Lennon, D.P.; Haynesworth, S.E.; Young, R.G.; Dennis, J.E.; Caplan, A.I. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp. Cell Res. 1995, 219, 211–222. [Google Scholar] [CrossRef]
- Carpentier, G. ImageJ Contribution: Angiogenesis Analyzer. ImageJ News, 5 October 2012; p. 1. [Google Scholar]
- Honda, M.; Aizawa, M. Preliminary Study for co-culture of osteoblasts and endothelial cells to construct the regenerative bone. Key Eng. Mater. 2017, 758, 269–272. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef]
- Hashida, Y.; Nakahama, K.; Shimizu, K.; Akiyama, M.; Harada, K.; Morita, I. Communication-dependent mineralization of osteoblasts via gap junctions. Bone 2014, 61, 19–26. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Reumann, M.K.; Boskey, A.; Wischmann, J.; von Eisenhart-Rothe, R.; Mayer-Kuckuk, P. Osteoblast migration in vertebrate bone. Biol. Rev. Camb. Philos. Soc. 2018, 93, 350–363. [Google Scholar] [CrossRef]
- Ichida, M.; Yui, Y.; Yoshioka, K.; Tanaka, T.; Wakamatsu, T.; Yoshikawa, H.; Itoh, K. Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Lett. 2011, 585, 4018–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koduru, S.V.; Leberfinger, A.N.; Pasic, D.; Forghani, A.; Lince, S.; Hayes, D.J.; Ozbolat, I.T.; Ravnic, D.J. Cellular based strategies for microvascular engineering. Stem Cell Rev. 2019, 15, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005, 90, 315–326. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, K.M.; Kim, E.J.; Jang, W.G. Hypothermia-induced RNA-binding motif protein 3 (RBM3) stimulates osteoblast differentiation via the ERK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 498, 459–465. [Google Scholar] [CrossRef]
- Aisha, M.D.; Nor-Ashikin, M.N.; Sharaniza, A.B.; Nawawi, H.M.; Kapitonova, M.Y.; Froemming, G.R. Short-term moderate hypothermia stimulates alkaline phosphatase activity and osteocalcin expression in osteoblasts by upregulating Runx2 and osterix in vitro. Exp. Cell Res. 2014, 326, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Buhrer, C.; Wellmann, S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell. Mol. Life Sci. 2016, 73, 3839–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef] [Green Version]
- Matz, J.M.; Blake, M.J.; Tatelman, H.M.; Lavoi, K.P.; Holbrook, N.J. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am. J. Physiol. 1995, 269, R38–R47. [Google Scholar] [CrossRef]
- Uchida, C.; Gee, E.; Ispanovic, E.; Haas, T.L. JNK as a positive regulator of angiogenic potential in endothelial cells. Cell Biol. Int. 2008, 32, 769–776. [Google Scholar] [CrossRef]
- Guillotin, B.; Bareille, R.; Bourget, C.; Bordenave, L.; Amedee, J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone 2008, 42, 1080–1091. [Google Scholar] [CrossRef]
- Lampert, F.M.; Simunovic, F.; Finkenzeller, G.; Pfeifer, D.; Stark, G.B.; Winninger, O.; Steiner, D. Transcriptomic changes in osteoblasts following endothelial cell-cocultivation suggest a role of extracellular matrix in cellular interaction. J. Cell. Biochem. 2016, 117, 1869–1879. [Google Scholar] [CrossRef]
- Simunovic, F.; Steiner, D.; Pfeifer, D.; Stark, G.B.; Finkenzeller, G.; Lampert, F. Increased extracellular matrix and proangiogenic factor transcription in endothelial cells after cocultivation with primary human osteoblasts. J. Cell. Biochem. 2013, 114, 1584–1594. [Google Scholar] [CrossRef]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Dyszkiewicz-Konwinska, M.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Bruska, M.; Izycki, D.; et al. Human Umbilical Vein Endothelial Cells (HUVECs) Co-culture with osteogenic cells: From molecular communication to engineering prevascularised bone grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef] [Green Version]
- Clarkin, C.E.; Garonna, E.; Pitsillides, A.A.; Wheeler-Jones, C.P. Heterotypic contact reveals a COX-2-mediated suppression of osteoblast differentiation by endothelial cells: A negative modulatory role for prostanoids in VEGF-mediated cell: Cell communication? Exp. Cell Res. 2008, 314, 3152–3161. [Google Scholar] [CrossRef]
- Clarkin, C.E.; Emery, R.J.; Pitsillides, A.A.; Wheeler-Jones, C.P. Evaluation of VEGF-mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J. Cell. Physiol. 2008, 214, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.H.; St-Pierre, J.P.; Stevens, M.M. Tissue engineering and regenerative medicine: A year in review. Tissue Eng. Part B Rev. 2014, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inomata, K.; Honda, M. Moderate Hypothermia Has the Potential to Reveal the Dominant/Submissive Relationship in a Co-Culture System Consisting of Osteoblasts and Endothelial Cells. Micro 2021, 1, 181-193. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1020014
Inomata K, Honda M. Moderate Hypothermia Has the Potential to Reveal the Dominant/Submissive Relationship in a Co-Culture System Consisting of Osteoblasts and Endothelial Cells. Micro. 2021; 1(2):181-193. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1020014
Chicago/Turabian StyleInomata, Kouki, and Michiyo Honda. 2021. "Moderate Hypothermia Has the Potential to Reveal the Dominant/Submissive Relationship in a Co-Culture System Consisting of Osteoblasts and Endothelial Cells" Micro 1, no. 2: 181-193. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1020014