Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation
Abstract
:1. Introduction
2. Morphological Characteristics of the Human Umbilical Cord (HUC)
2.1. Vessels of HUC
2.2. Vascular Endothelium
3. Isolation of Endothelial Cells of HUC
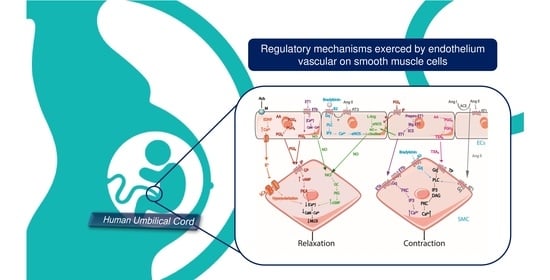
4. Regulation of Vascular Tone by Endothelial Cells
4.1. Nitric Oxide
4.2. Endothelin 1
4.3. Prostacyclin
4.4. Endothelium-Derived Hyperpolarization Factor
4.5. Thromboxane A2
4.6. Angiotensin II
4.7. Bradykinin
5. Endothelial Dysfunction
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.; Gossen, M.; Lendlein, A.; Jung, F. Venous and Arterial Endothelial Cells from Human Umbilical Cords: Potential Cell Sources for Cardiovascular Research. Int. J. Mol. Sci. 2021, 22, 978. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Maamoun, H.; Abdelsalam, S.S.; Zeidan, A.; Korashy, H.M.; Agouni, A. Endoplasmic Reticulum Stress: A Critical Molecular Driver of Endothelial Dysfunction and Cardiovascular Disturbances Associated with Diabetes. Int. J. Mol. Sci. 2019, 20, 1658. [Google Scholar] [CrossRef] [Green Version]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campesi, I.; Franconi, F.; Montella, A.; Dessole, S.; Capobianco, G. Human Umbilical Cord: Information Mine in Sex-Specific Medicine. Life 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Dey, S.K.; Kundu, S. Functional implications of vascular endothelium in regulation of endothelial nitric oxide synthesis to control blood pressure and cardiac functions. Life Sci. 2020, 259, 118377. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Radomski, M.W. The nitric oxide-endothelin-1 connection. Heart Fail. Rev. 2003, 8, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Storch, A.S.; de Mattos, J.D.; Alves, R.; Galdino, I.d.S.; Rocha, H.N.M. Methods of Endothelial Function Assessment: Description and Applications. Int. J. Cardiovasc. Sci. 2017, 30, 262–273. [Google Scholar] [CrossRef]
- Martin de Llano, J.J.; Fuertes, G.; Garcia-Vicent, C.; Torro, I.; Fayos, J.L.; Lurbe, E. Procedure to consistently obtain endothelial and smooth muscle cell cultures from umbilical cord vessels. Transl. Res. 2007, 149, 1–9. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. Human Umbilical Artery Smooth Muscle Cells: Vascular Function and Clinical Importance. In Horizons in World Cardiovascular Research; Nova Science Publishers: New York, NY, USA, 2019; Volume 16, pp. 81–137. [Google Scholar]
- Yampolsky, M.; Salafia, C.M.; Shlakhter, O.; Haas, D.; Eucker, B.; Thorp, J. Modeling the variability of shapes of a human placenta. Placenta 2008, 29, 790–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshiri, M.; Zaidi, S.F.; Robinson, T.J.; Bhargava, P.; Siebert, J.R.; Dubinsky, T.J.; Katz, D.S. Comprehensive imaging review of abnormalities of the umbilical cord. Radiographics 2014, 34, 179–196. [Google Scholar] [CrossRef]
- Chillakuru, S.; Velichety, S.D.; Rajagopalan, V. Human umbilical cord and its vessels: A histomorphometric study in difference severity of hypertensive disorders of pregnancy. Anat. Cell Biol. 2020, 53, 68–75. [Google Scholar] [CrossRef]
- Bosselmann, S.; Mielke, G. Sonographic Assessment of the Umbilical Cord. Geburtshilfe Frauenheilkd. 2015, 75, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiz, A.A.; Rahime, B.; Keskin, H.L.; Esra, A.K. Positive correlation between the quantity of Wharton’s jelly in the umbilical cord and birth weight. Taiwan. J. Obstet. Gynecol. 2011, 50, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, V.L.; Dodson, R.B. Bioengineering aspects of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S108–S113. [Google Scholar] [CrossRef]
- Kellow, Z.S.; Feldstein, V.A. Ultrasound of the placenta and umbilical cord: A review. Ultrasound Q. 2011, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, W. Time for a more detailed prenatal examination of the umbilical cord? Ultrasound Obs. Gynecol. 1999, 13, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Spurway, J.; Logan, P.; Pak, S. The development, structure and blood flow within the umbilical cord with particular reference to the venous system. Australas. J. Ultrasound Med. 2012, 15, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; McGrath, B.C.; Bai, Y.; Tang, X.; Cavener, D.R. PERK regulates Gq protein-coupled intracellular Ca2+ dynamics in primary cortical neurons. Mol. Brain 2016, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M. Endothelium and control of vascular function. State of the Art lecture. Hypertension 1989, 13, 658–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetin, A.; Kukner, A.; Ozturk, F. Ultrastructure of human umbilical vessels in pre-eclampsia. J. Matern.-Fetal Neonatal Med. 2002, 12, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Rockelein, G.; Schneider, R. Three-dimensional analysis of the tunica media of umbilical arteries. Scanning electron microscopy study. Z. Geburtshilfe Perinatol. 1992, 196, 266–272. [Google Scholar] [PubMed]
- Meyer, W.W.; Rumpelt, H.J.; Yao, A.C.; Lind, J. Structure and closure mechanism of the human umbilical artery. Eur. J. Pediatr. 1978, 128, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, V.E.; Martinez-Gonzalez, B.; Quiroga-Garza, A.; Reyes-Hernandez, C.G.; de la Fuente-Villarreal, D.; de la Garza-Castro, O.; Guzman-Lopez, S.; Elizondo-Omana, R.E. Human Umbilical Vessels: Choosing the Optimal Decellularization Method. ASAIO J. 2018, 64, 575–580. [Google Scholar] [CrossRef] [PubMed]
- DeFreitas, M.J.; Mathur, D.; Seeherunvong, W.; Cano, T.; Katsoufis, C.P.; Duara, S.; Yasin, S.; Zilleruelo, G.; Rodriguez, M.M.; Abitbol, C.L. Umbilical artery histomorphometry: A link between the intrauterine environment and kidney development. J. Dev. Orig. Health Dis. 2017, 8, 349–356. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91. [Google Scholar] [PubMed]
- Vanhoutte, P.M. COX-1 and vascular disease. Clin. Pharmacol. Ther. 2009, 86, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, P.S.; Joseph, L.B.; Titterington, L.; Stephens, R.E. Methods for the Initiation and Maintenance of Human Endothelial Cell Culture. Vasc. Surg. 1987, 21, 391–400. [Google Scholar] [CrossRef]
- Siow, R.C. Culture of human endothelial cells from umbilical veins. Methods Mol. Biol. 2012, 806, 265–274. [Google Scholar]
- Lewis, W.H. Endothelium in tissue cultures. Am. J. Anat. 1922, 30, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y. The human endothelial cell in tissue culture. Z. Zellforsch. Mikrosk. Anat. 1963, 60, 69–79. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Cotran, R.S.; Folkman, J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 1974, 60, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Kaplanski, G.; Grès, S.; Farnarier, C.; Bongrand, P. Endothelial cell culture: Protocol to obtain and cultivate human umbilical endothelial cells. J. Immunol. Methods 2001, 254, 183–190. [Google Scholar] [CrossRef]
- Ulrich-Merzenich, G.; Metzner, C.; Bhonde, R.R.; Malsch, G.; Schiermeyer, B.; Vetter, H. Simultaneous Isolation of Endothelial and Smooth Muscle Cells from Human Umbilical Artery or Vein and Their Growth Response to Low-Density Lipoproteins. In Vitr. Cell. Dev. Biol.-Anim. 2002, 38, 265–272. [Google Scholar] [CrossRef]
- Yang, S.J.; Son, J.K.; Hong, S.J.; Lee, N.E.; Shin, D.Y.; Park, S.H.; An, S.B.; Sung, Y.C.; Park, J.B.; Yang, H.M.; et al. Ectopic vascularized bone formation by human umbilical cord-derived mesenchymal stromal cells expressing bone morphogenetic factor-2 and endothelial cells. Biochem. Biophys. Res. Commun. 2018, 504, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Evensen, S.A.; Elgjo, R.F.; Vefling, A. Human fetal endothelial cells in cluture. Scand. J. Haematol. 1975, 14, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef]
- Cheung, A.L. Isolation and culture of human umbilical vein endothelial cells (HUVEC). Curr. Protoc. Microbiol. 2007, 4. [Google Scholar] [CrossRef]
- Kunkanjanawan, H.; Kunkanjanawan, T.; Khemarangsan, V.; Yodsheewan, R.; Theerakittayakorn, K.; Parnpai, R. A Xeno-Free Strategy for Derivation of Human Umbilical Vein Endothelial Cells and Wharton’s Jelly Derived Mesenchymal Stromal Cells: A Feasibility Study toward Personal Cell and Vascular Based Therapy. Stem Cells Int. 2020, 2020, 8832052. [Google Scholar] [CrossRef]
- Larrivee, B.; Karsan, A. Isolation and culture of primary endothelial cells. Methods Mol. Biol. 2005, 290, 315–329. [Google Scholar] [PubMed]
- Pipino, C.; Shah, H.; Prudente, S.; Di Pietro, N.; Zeng, L.; Park, K.; Trischitta, V.; Pennathur, S.; Pandolfi, A.; Doria, A. Association of the 1q25 Diabetes-Specific Coronary Heart Disease Locus With Alterations of the gamma-Glutamyl Cycle and Increased Methylglyoxal Levels in Endothelial Cells. Diabetes 2020, 69, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, L.; Burlakov, J.; Hass, S.; von Kaisenberg, C.; von Versen-Hoynck, F. Impaired functional capacity of fetal endothelial cells in preeclampsia. PLoS ONE 2017, 12, e0178340. [Google Scholar] [CrossRef]
- Amrithraj, A.I.; Kodali, A.; Nguyen, L.; Teo AK, K.; Chang, C.W.; Karnani, N.; Ng, K.L.; Gluckman, P.D.; Chong, Y.S.; Stunkel, W. Gestational Diabetes Alters Functions in Offspring’s Umbilical Cord Cells with Implications for Cardiovascular Health. Endocrinology 2017, 158, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Psefteli, P.M.; Kitscha, P.; Vizcay, G.; Fleck, R.; Chapple, S.J.; Mann, G.E.; Fowler, M.; Siow, R.C. Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells. Redox Biol. 2021, 38, 101816. [Google Scholar] [CrossRef]
- Bachetti, T.; Morbidelli, L. Endothelial cells in culture: A model for studying vascular functions. Pharmacol. Res. 2000, 42, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Provitera, L.; Cavallaro, G.; Griggio, A.; Raffaeli, G.; Amodeo, I.; Gulden, S.; Lattuada, D.; Ercoli, G.; Lonati, C.; Tomaselli, A.; et al. Cyclic nucleotide-dependent relaxation in human umbilical vessels. J. Physiol. Pharmacol. 2019, 70, 619–630. [Google Scholar]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre MP, A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide mitigates TNF-alpha induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes Metab. Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.S.; Tiwari, S.; Bhonde, R.R. Simultaneous isolation of vascular endothelial cells and mesenchymal stem cells from the human umbilical cord. In Vitr. Cell. Dev. Biol.-Anim. 2009, 45, 23–27. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, L.; Molgaard, H.V.; Robertson, D.; Bradley, N.J.; Monaghan, P.; Delia, D.; Sutherland, D.R.; Baker, M.A.; Greaves, M.F. Expression of the CD34 gene in vascular endothelial cells. Blood 1990, 75, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutin, M.; Dignat-George, F.; Sampol, J. Immunologic phenotype of cultured endothelial cells: Quantitative analysis of cell surface molecules. Tissue Antigens 1997, 50, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.E.; Vaporciyan, A.A.; Bonish, B.K.; Jones, M.L.; Johnson, K.J.; Glovsky, M.M.; Eddy, S.M.; Ward, P.A. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 1994, 94, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Pei, H.; Wang, S.; Zhang, B.; Fan, Z.; Liu, Y.; Xie, X.; Yang, Z.; Xu, L.; Jia, Y.; et al. Arterial endothelium creates a permissive niche for expansion of human cord blood hematopoietic stem and progenitor cells. Stem Cell Res. Ther. 2020, 11, 358. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef]
- Chi, J.T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA. 2003, 100, 10623–10628. [Google Scholar] [CrossRef] [Green Version]
- Mann, G.E.; Pearson, J.D.; Sheriff, C.J.; Toothill, V.J. Expression of amino acid transport systems in cultured human umbilical vein endothelial cells. J. Physiol. 1989, 410, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Sobrevia, L.; Cesare, P.; Yudilevich, D.L.; Mann, G.E. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: Association with membrane hyperpolarization. J. Physiol. 1995, 489, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahabeleshwar, G.H.; Somanath, P.R.; Byzova, T.V. Methods for isolation of endothelial and smooth muscle cells and in vitro proliferation assays. Methods Mol. Med. 2006, 129, 197–208. [Google Scholar] [PubMed]
- Crampton, S.P.; Davis, J.; Hughes, C.C. Isolation of human umbilical vein endothelial cells (HUVEC). J. Vis. Exp. 2007, 3, e183. [Google Scholar]
- Casanello, P.; Krause, B.; Torres, E.; Gallardo, V.; González, M.; Prieto, C.; Escudero, C.; Farías, M.; Sobrevia, L. Reduced l-arginine transport and nitric oxide synthesis in human umbilical vein endothelial cells from intrauterine growth restriction pregnancies is not further altered by hypoxia. Placenta 2009, 30, 625–633. [Google Scholar] [CrossRef]
- Krause, B.J.; Prieto, C.P.; Munoz-Urrutia, E.; San Martin, S.; Sobrevia, L.; Casanello, P. Role of arginase-2 and eNOS in the differential vascular reactivity and hypoxia-induced endothelial response in umbilical arteries and veins. Placenta 2012, 33, 360–366. [Google Scholar] [CrossRef]
- Pang, H. Make Human Umbilical Vein Endothelial Cells from Cords. Bio-Protocol 2012, 2, e204. [Google Scholar] [CrossRef]
- Lattuada, D.; Roda, B.; Pignatari, C.; Magni, R.; Colombo, F.; Cattaneo, A.; Zattoni, A.; Cetin, I.; Reschiglian, P.; Bolis, G. A tag-less method for direct isolation of human umbilical vein endothelial cells by gravitational field-flow fractionation. Anal. Bioanal. Chem. 2013, 405, 977–984. [Google Scholar] [CrossRef]
- Lei, J.; Peng, S.; Samuel, S.B.; Zhang, S.; Wu, Y.; Wang, P.; Li, Y.F.; Liu, H. A simple and biosafe method for isolation of human umbilical vein endothelial cells. Anal. Biochem. 2016, 508, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.J.; Hernandez, C.; Caniuguir, A.; Vasquez-Devaud, P.; Carrasco-Wong, I.; Uauy, R.; Casanello, P. Arginase-2 is cooperatively up-regulated by nitric oxide and histone deacetylase inhibition in human umbilical artery endothelial cells. Biochem. Pharmacol. 2016, 99, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Suhaila, R.N.; Safuan, S. Isolation Methods and Culture Conditions of Human Umbilical Vein Endothelial Cells from Malaysian Women. Sains Malays. 2017, 46, 463–468. [Google Scholar] [CrossRef]
- Thormodsson, F.R.; Olafsson, I.H.; Vilhjalmsson, D.T. Preparation and Culturing of Human Primary Vascular Cells. Methods Mol. Biol. 2018, 1779, 355–369. [Google Scholar]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Izumi, H.; Makino, Y.; Shirakawa, K.; Garfield, R.E. Role of nitric oxide on vasorelaxation in human umbilical artery. Am. J. Obstet. Gynecol. 1995, 172, 1477–1484. [Google Scholar] [CrossRef]
- Izumi, H.; Makino, Y.; Mohtai, H.; Shirakawa, K.; Garfield, R.E. Comparison of nitric oxide and prostacyclin in endothelium-dependent vasorelaxation of human umbilical artery at midgestation. Am. J. Obstet. Gynecol. 1996, 175, 375–381. [Google Scholar] [CrossRef]
- Lovren, F.; Triggle, C. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br. J. Pharmacol. 2000, 131, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkranz-Weiss, P.; Sessa, W.C.; Milstien, S.; Kaufman, S.; Watson, C.A.; Pober, J.S. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J. Clin. Investig. J. 1994, 93, 2236–2243. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.M.; Chen, T.L.; Lin, Y.L.; Chen, T.G.; Tai, Y.T. Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells by down-regulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit. Care Med. 2005, 33, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, N.A.; Abdullah Mahdy, Z. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci. World J. 2014, 2014, 169370. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Bras-Silva, C.; Cerdeira, A.S.; Leite-Moreira, A.F. Cardiovascular endothelins: Essential regulators of cardiovascular homeostasis. Pharmacol. Ther. 2006, 111, 508–531. [Google Scholar] [CrossRef]
- Sanchez, A.; Martinez, P.; Munoz, M.; Benedito, S.; Garcia-Sacristan, A.; Hernandez, M.; Prieto, D. Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: Role of ET(A) and ET(B) receptors. Br. J. Pharmacol. 2014, 171, 5682–5695. [Google Scholar] [CrossRef] [Green Version]
- Enevoldsen, F.C.; Sahana, J.; Wehland, M.; Grimm, D.; Infanger, M.; Kruger, M. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. J. Clin. Med. 2020, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Guan, Z.; VanBeusecum, J.P.; Inscho, E.W. Endothelin and the renal microcirculation. Semin. Nephrol. 2015, 35, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, S.A.; El-Mas, M.M. Endothelin ETA receptor antagonism in cardiovascular disease. Eur. J. Pharmacol. 2014, 737, 210–213. [Google Scholar] [CrossRef]
- Thorin, E.; Webb, D.J. Endothelium-derived endothelin-1. Pflug. Arch. 2010, 459, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Khimji, A.K.; Rockey, D.C. Endothelin--biology and disease. Cell. Signal. 2010, 22, 1615–1625. [Google Scholar] [CrossRef]
- Pernow, J.; Shemyakin, A.; Bohm, F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci. 2012, 91, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Majed, B.H.; Khalil, R.A. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 2012, 64, 540–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabito Colafella, K.M.; Neuman, R.I.; Visser, W.; Danser AH, J.; Versmissen, J. Aspirin for the prevention and treatment of pre-eclampsia: A matter of COX-1 and/or COX-2 inhibition? Basic Clin. Pharmacol. Toxicol. 2020, 127, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Villar, I.C.; Francis, S.; Webb, A.; Hobbs, A.J.; Ahluwalia, A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 2006, 70, 840–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eivers, S.B.; Kinsella, B.T. Regulated expression of the prostacyclin receptor (IP) gene by androgens within the vasculature: Combined role for androgens and serum cholesterol. Biochim. Biophys. Acta 2016, 1859, 1333–1351. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011, 164, 894–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, S.W.S. The vascular impact of IP-TP receptor interactions. Acta Physiol. 2020, 231, e13577. [Google Scholar]
- Lim, H.; Dey, S.K. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 2002, 143, 3207–3210. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, K.; Sintetos, A.; Ramwell, P. The effect of prostacyclin on the human umbilical artery. Prostaglandins 1978, 15, 1035–1044. [Google Scholar] [CrossRef]
- Klockenbusch, W.; Braun, M.S.; Schröder, H.; Heckenberger, R.E.; Strobach, H.; Schrör, K. Prostacyclin rather than nitric oxide lowers human umbilical artery tone in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 47, 109–115. [Google Scholar] [CrossRef]
- Estrada-García, L.; Carrera-Rotllan, J.; Puig-Parellada, P. Effects of oxidative stress and antioxidant treatments on eicosanoid synthesis and lipid peroxidation in long term human umbilical vein endothelial cells culture. Prostaglandins Other Lipid Mediat. 2002, 67, 13–25. [Google Scholar] [CrossRef]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, Y.; Zhang, Y.; Zhang, Y.; Li, H.; Zheng, Q.; Li, N.; Tang, J.; Xu, Z. New views on endothelial dysfunction in gestational hypertension and potential therapy targets. Drug Discov. Today 2021, 26, 1420–1436. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Durante, W.; Sowers, J.R. Endothelium-Derived Hyperpolarizing Factors: A Potential Therapeutic Target for Vascular Dysfunction in Obesity and Insulin Resistance. Diabetes 2016, 65, 2118–2120. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, H.; Godo, S. Nitric oxide and endothelium-dependent hyperpolarization mediated by hydrogen peroxide in health and disease. Basic Clin. Pharmacol. Toxicol. 2020, 127, 92–101. [Google Scholar] [CrossRef]
- Heptinstall, S. Antiplatelet Agents: Current and Novel. In Antiplatelet and Anticoagulation Therapy; Ferro, A., Garcia, D.A., Eds.; Springer: London, UK, 2013; pp. 1–44. [Google Scholar]
- Wu, Z.; Yao, H.; Xu, H.; Wang, Y.; Hu, W.; Lou, G.; Zhang, L.; Huang, C.; Jiang, C.; Zhou, S.; et al. Inhibition of eNOS by L-NAME resulting in rat hind limb developmental defects through PFKFB3 mediated angiogenetic pathway. Sci. Rep. 2020, 10, 16754. [Google Scholar] [CrossRef]
- Suksawat, M.; Techasen, A.; Namwat, N.; Boonsong, T.; Titapun, A.; Ungarreevittaya, P.; Yongvanit, P.; Loilome, W. Inhibition of endothelial nitric oxide synthase in cholangiocarcinoma cell lines—A new strategy for therapy. FEBS Open Bio 2018, 8, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Luksha, L.; Agewall, S.; Kublickiene, K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 2009, 202, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br. J. Pharmacol. 2009, 156, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Martinez-Lemus, L.A.; Zhang, C. Endothelium-derived hyperpolarizing factor and diabetes. World J. Cardiol. 2011, 3, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018, 134, 32–37. [Google Scholar] [CrossRef]
- Sellers, M.M.; Stallone, J.N. Sympathy for the devil: The role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1978–H1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodelsson, G.; Maršál, K.; Stjernquist, M. Reduced contractile effect of endothelin-1 and noradrenalin in human umbilical artery from pregnancies with abnormal umbilical artery flow velocity waveforms. Early Hum. Dev. 1995, 42, 15–28. [Google Scholar] [CrossRef]
- Ellinsworth, D.C.; Shukla, N.; Fleming, I.; Jeremy, J.Y. Interactions between thromboxane A(2), thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc. Res. 2014, 102, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Kakami, M.; Noguchi, E.; Kobayashi, T.; Kamata, K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1480–H1490. [Google Scholar] [CrossRef]
- Matsumoto, T.; Noguchi, E.; Ishida, K.; Kobayashi, T.; Yamada, N.; Kamata, K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1165–H1176. [Google Scholar] [CrossRef] [Green Version]
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Bonaventura, D.; Tirapelli, L.F.; de Oliveira, A.M. Mechanisms underlying the vascular actions of endothelin 1, angiotensin II and bradykinin in the rat carotid. Pharmacology 2009, 84, 111–126. [Google Scholar] [CrossRef]
- de Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar] [PubMed]
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 2014, 16, 431. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Faria-Costa, G.; Leite-Moreira, A.; Henriques-Coelho, T. Cardiovascular effects of the angiotensin type 2 receptor. Rev. Port. Cardiol. 2014, 33, 439–449. [Google Scholar] [CrossRef]
- Masi, S.; Uliana, M.; Virdis, A. Angiotensin II and vascular damage in hypertension: Role of oxidative stress and sympathetic activation. Vasc. Pharm. 2019, 115, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Schena, M.; Mulatero, P.; Schiavone, D.; Mengozzi, G.; Tesio, L.; Chiandussi, L.; Veglio, F. Vasoactive hormones induce nitric oxide synthase mRNA expression and nitric oxide production in human endothelial cells and monocytes. Am. J. Hypertens. 1999, 12, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Radenkovic, M.; Grbovic, L.; Radunovic, N.; Momcilov, P. Pharmacological evaluation of bradykinin effect on human umbilical artery in normal, hypertensive and diabetic pregnancy. Pharmacol. Rep. 2007, 59, 64–73. [Google Scholar]
- Hornig, B.; Drexler, H. Endothelial function and bradykinin in humans. Drugs 1997, 54, 42–47. [Google Scholar] [CrossRef]
- Moreau, M.E.; Garbacki, N.; Molinaro, G.; Brown, N.J.; Marceau, F.; Adam, A. The kallikrein-kinin system: Current and future pharmacological targets. J. Pharmacol. Sci. 2005, 99, 6–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koumbadinga, G.A.; Desormeaux, A.; Adam, A.; Marceau, F. Effect of interferon-gamma on inflammatory cytokine-induced bradykinin B1 receptor expression in human vascular cells. Eur. J. Pharmacol. 2010, 647, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marceau, F.; Bachelard, H.; Bouthillier, J.; Fortin, J.P.; Morissette, G.; Bawolak, M.T.; Charest-Morin, X.; Gera, L. Bradykinin receptors: Agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. Int. Immunopharmacol. 2020, 82, 106305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tom, B.; de Vries, R.; Saxena, P.R.; Danser, A.H. Bradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension 2001, 38, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Takano, M.; Matsuyama, S. Intracellular and nuclear bradykinin B2 receptors. Eur. J. Pharmacol. 2014, 732, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Rhaleb, I.A.; Kassem, K.M.; Rhaleb, N.E. Role of Kinins in Hypertension and Heart Failure. Pharmaceuticals 2020, 13, 347. [Google Scholar] [CrossRef]
- Bai, B.; Liu, L.; Zhang, N.; Wang, C.; Jiang, Y.; Chen, J. Heterodimerization of human apelin and bradykinin 1 receptors: Novel signal transduction characteristics. Cell. Signal. 2014, 26, 1549–1559. [Google Scholar] [CrossRef]
- Murphey, L. Contribution of bradykinin to the cardioprotective effects of ACE inhibitors. Eur. Heart J. Suppl. 2003, 5, A37–A41. [Google Scholar] [CrossRef] [Green Version]
- Conlon, J.M. The kallikrein-kinin system: Evolution of form and function. Ann. N. Y. Acad. Sci. 1998, 839, 1–8. [Google Scholar] [CrossRef]
- Nowak, W.; Errasti, A.E.; Armesto, A.R.; Santin Velazque, N.L.; Rothlin, R.P. Endothelial angiotensin-converting enzyme and neutral endopeptidase in isolated human umbilical vein: An effective bradykinin inactivation pathway. Eur. J. Pharmacol. 2011, 667, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Bortolotto, L. Unraveling the Pivotal Role of Bradykinin in ACE Inhibitor Activity. Am. J. Cardiovasc. Drugs 2016, 16, 309–321. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharm. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Grover-Paez, F.; Zavalza-Gomez, A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009, 84, 1–10. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Wang, Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediat. Inflamm. 2017, 2017, 8135934. [Google Scholar] [CrossRef]
- Knapp, M.; Tu, X.; Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharm. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef]
- Radenkovic, M.; Stojanovic, M.; Nesic, I.M.; Prostran, M. Angiotensin receptor blockers & endothelial dysfunction: Possible correlation & therapeutic implications. Indian J. Med. Res. 2016, 144, 154–168. [Google Scholar] [PubMed]
- Echeverria, C.; Eltit, F.; Santibanez, J.F.; Gatica, S.; Cabello-Verrugio, C.; Simon, F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165414. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Aranguren, L.C.; Prada, C.E.; Riano-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Nakhla, S.; Makris, A.; Hennessy, A. TNF-α inhibits trophoblast integration into endothelial cellular networks. Placenta 2011, 32, 241–246. [Google Scholar] [CrossRef] [PubMed]

| Studies Performed | Digestive Enzyme | Medium Used | Centrifugation Conditions | Coating | Cells | Protocol Based on | Observations |
|---|---|---|---|---|---|---|---|
| Maruyama et al. (1963) [38] | 0.2% Trypsin | YLH | - | Rat-tail Collagen (coverslip) | HUVECs | - | The cells were cultured in sheets |
| Jaffe et al. (1973) [39] | 0.2% Collagenase | TC 199 | 250 g 10 min | - | HUVECs | Based on Maruyama et al. | ECs differentiation based on morphologic and immunologic criteria |
| Mann et al. (1989) [65] | Collagenase type II | M199 | - | 1% Gelatin | HUVECs | Based on Jaffe et al. | ECs were cultured on microcarrier beads |
| Sobrevia et al. (1995) [66] | Collagenase | M199 | - | - | HUVECs | - | HUC from gestational diabetic pregnancies |
| Marin et al. (2001) [41] | Collagenase A | M199 | 1500 r/min 5 min | 1% Gelatin | HUVECs | - | Three different cords were used to limit the variability of ECs |
| Ulrich-mersenich et al. (2002) [42] | Dispase II and Collagenase tipo IV | M199 | 1500 rpm 15 min | Fibronectin | HUVECS and HUAECS | Based on Ko et al. (1995) | Isolation of ECs and SMC from the same vessel of HUC obtain the best results |
| Larrivee et al. (2005) [48] | Collagenase A | MCDB 131 | 300 g 5 min | 0.2% Gelatin | HUVECs | - | ECs successful cryopreservation |
| Mahabeleshwar et al. (2006) [67] | 0.1% Collagenase | Endothelial cell growth medium | 320 g 10 min | Gelatin | HUVECs | - | Obtention pure cultures of ECs |
| Baudin et al. (2007) [45] | 0.2% Collagenase | M199 | 750 g 10 min | Fibronectin | HUVECs | Based on Jaffe et al. | HUVECs can keep phenotypic features in free serum medium for up 12 h |
| Crampton et al. (2007) [68] | Collagenase | M199 | 1200 rpm 5 min | Gelatin | HUVECs | - | Used in angiogenesis assay |
| Cheung et al. (2007) [46] | 0.1% Collagenase | M199 | 250 g 10 min Room temperature | Gelatin | HUVECs | - | Confluent T25 flask was obtained in 4–5 days. Cells beyond the 6th passage should be discarded. |
| Martin de llano et al. (2007) [11] | Collagenase type I and Dispase | M200 or M231 | 10 g 10 min | Fibronectin | HUVECs and HUAECs | - | Birth weight does not have influence on the time request to obtain grown cultures |
| Casanello et al. (2009) [69] | Collagenase | M199 | - | - | HUVECs | Based on Sobrevia et al. | ECs maintain the protein expression until 5th passage |
| Kadam et al. (2009) [57] | Dispase II and Collagenase type IV | M199 | 1500 rpm 15 min | Fibronectin | HUVECS and hUCMSCs | Based on Ulrich-mersenich et al. | Two-steps protocol for the simultaneous isolation of ECs and hUCMSCs |
| Krause et al. (2012) [70] | Collagenase | M199 | - | - | HUVECs and HUAECs | Based on Casanello et al. | ECs are responsive to hypoxia |
| Pang et al. (2012) [71] | Collagenase | M199 | 1000 g 5 min | - | HUVECs | Based on Jaffe et al. | ECs are responsive to cytokine stimulation |
| Siow et al. (2012) [36] | Collagenase | M199 | 1000 rpm 5 min | Gelatin | HUVECs | Based on Jaffe et al. | Confluent culture of ECs after 2–3 days will start to detach and decrease viability |
| Lattuada et al. (2013) [72] | 0.1% Collagenase A | M199 | 463 g 15 min | - | HUVECs | Based on Jaffe et al. | Gravitational field-flow fractionation is the new method to easy isolate ECs from HUC |
| Lei et al. (2016) [73] | 0.2% Collagenasee | M199 | 800 g 5 min | Gelatin | HUVECs | - | Used a new instrument for insertion of HUC |
| Krause et al. (2016) [74] | Collagenase | M199 | - | - | HUAECs | Based on Krause et al. (2012) | ECs pure culture can be used for protein expression |
| Amrithraj et al. (2017) [51] | Collagenase | EGM-2 | 1200 rpm 5 min | Gelatin | HUVECs | Based on Crampton et al. | ECs culture from gestational diabetic pregnancies maintain metabolic and molecular imprints of maternal hyperglycemia |
| Brodowaski et al. (2017) [50] | 0.2% Collagenase | EGM | - | - | HUVEC | Based on Jaffe et al. | EC culture from preeclamptic women |
| Di tomo et al. (2017) [55] | Collagenase 1 | M199 | - | 1.5% Gelatin | HUVECs | - | EC culture was obtained with explants |
| Suhaila et al. (2017) [75] | Collagenase type I | M199 | 1500 rpm 5 min | They tested various concentrations of gelatin | HUVECs | - | The 0.2% gelatin obtain the best results |
| Thormodsson et al. (2018) [76] | Collagenase | M199 | 140 g 5 min | - | HUVECS and HUAECs | - | Using NunclonTM Δ T-25 flasks: the cells attach without any problem |
| Yang et al. (2018) [43] | 0.05% Collagenase I | EGM-2 | 250 g 5 min | Fibronectin | HUVECs | Based on Jaffe et al. | The cells were negative for CD34, CD45, and human leukocyte antigen–DR isotype (HLA-DR) |
| Provitera et al. (2019) [54] | 0.1% Collagenase A | M199 | 463 g 15 min | - | HUAECs and HUVECs | Based on Lattuada et al. | Cells were used at P0 |
| Pipino et al. (2020) [49] | Collagenase type1A | DMEM | - | 1.5% Gelatin | HUVECs | Based on Di tomo et al. | Cells between the 3rd and 7th passages were used |
| Psefteli et al. (2021) [52] | 0.2% Collagenase | M199 | - | Gelatin | HUVECs | Based on Jaffe et al. | Cells between P0 and P3 were used for protein expression |
| Vasorelaxation | Vasoconstriction | Both Effects |
|---|---|---|
| NO (guanylyl cyclase) | TXA2 (TP receptor) | ET-1 (ETA and ETB receptor) |
| EDHF (potassium channels) | Bradykinin (B1 and B2 receptor) | |
| PGI2 (IP receptor) | ANGII (AT1 and AT2 receptor) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangana, C.; Lorigo, M.; Cairrao, E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics 2021, 1, 231-251. https://0-doi-org.brum.beds.ac.uk/10.3390/biologics1020015
Mangana C, Lorigo M, Cairrao E. Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation. Biologics. 2021; 1(2):231-251. https://0-doi-org.brum.beds.ac.uk/10.3390/biologics1020015
Chicago/Turabian StyleMangana, Carolina, Margarida Lorigo, and Elisa Cairrao. 2021. "Implications of Endothelial Cell-Mediated Dysfunctions in Vasomotor Tone Regulation" Biologics 1, no. 2: 231-251. https://0-doi-org.brum.beds.ac.uk/10.3390/biologics1020015