Effects of Memantine in Patients with Traumatic Brain Injury: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
- (1)
- (patients) target population must solely be adult patients with TBI;
- (2)
- (intervention) memantine as a monotherapy in the therapeutic arm;
- (3)
- (comparator interventions) placebo or standard treatment;
- (4)
- (outcomes) cognitive tests, GCS and serum neuron-specific enolase;
- (5)
- (methods-study design) RCTs;
- (6)
- (time or duration) no specified follow-up time.
2.3. Data Collection
2.4. Risk of Bias
2.5. Statisitical Analysis
3. Results
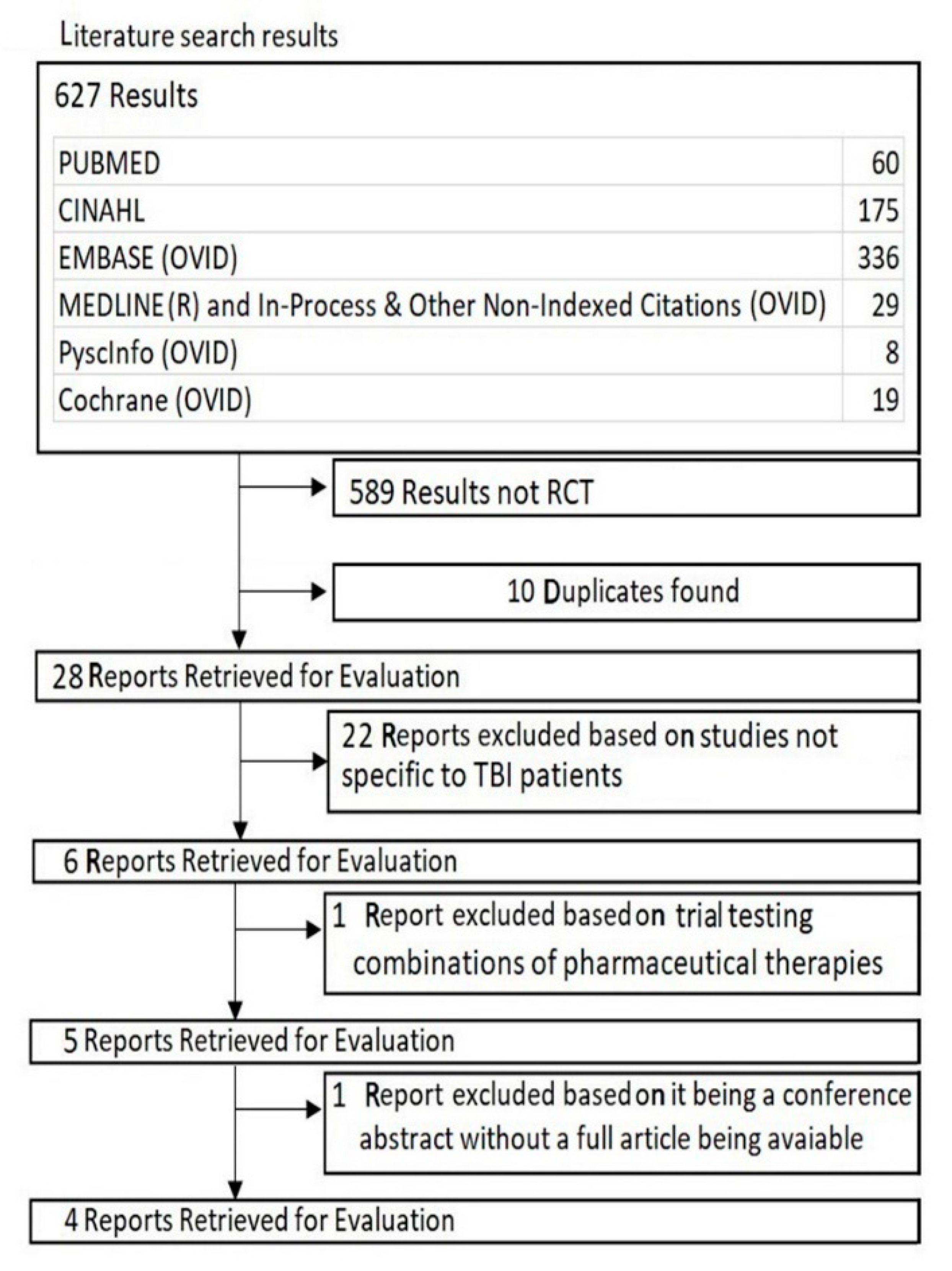
3.1. Study Selection
3.2. Study Characteristics
3.3. Results of Individual Studies
3.4. Synthesis of Results
3.5. Risk of Bias within Studies
3.6. Risk of Bias across Studies
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [Green Version]
- Te Ao, B.; Brown, P.; Tobias, M.; Ameratunga, S.; Barker-Collo, S.; Theadom, A.; McPherson, K.; Starkey, N.; Dowell, A.; Jones, K.; et al. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology 2014, 83, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Scholten, A.; Polinder, S.; Panneman, M.J.; Van Beeck, E.F.; Haagsma, J.A. Incidence and costs of bicycle-related traumatic brain injuries in the Netherlands. Accid. Anal. Prev. 2015, 81, 51–60. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W. Neurobehavioral sequelae of traumatic brain injury: Evaluation and management. World Psychiatry 2008, 7, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care. 2016, 20, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, J.; Costanzo, R.M.; Reiter, E.R. Head trauma and olfactory function. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 39–45. [Google Scholar] [CrossRef]
- Sen, N. An insight into the vision impairment following traumatic brain injury. Neurochem. Int. 2017, 111, 103–107. [Google Scholar] [CrossRef]
- Annegers, J.F.; Hauser, W.A.; Coan, S.P.; Rocca, W.A. A Population-Based Study of Seizures after Traumatic Brain Injuries. N. Engl. J. Med. 1998, 338, 20–24. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Held, K.; Wagner, P. Cognitive impairment following traumatic brain injury. Curr. Treatment Opt. Neurol. 2002, 4, 43–57. [Google Scholar] [CrossRef]
- Cardoso, M.G.D.F.; Faleiro, R.M.; De Paula, J.J.; Kummer, A.; Caramelli, P.; Teixeira, A.L.; De Souza, L.C.; De Miranda, A.S. Cognitive Impairment Following Acute Mild Traumatic Brain Injury. Front. Neurol. 2019, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Veeramuthu, V.; Narayanan, V.; Kuo, T.L.; Delano-Wood, L.; Chinna, K.; Bondi, M.W.; Vigneswaran, V.; Ganesan, D.; Ramli, N. Diffusion Tensor Imaging Parameters in Mild Traumatic Brain Injury and Its Correlation with Early Neuropsychological Impairment: A Longitudinal Study. J. Neurotrauma 2015, 32, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Gattu, R.; Kobeissy, F.; Welch, R.D.; O’Neil, B.J.; Woodard, J.L.; Ayaz, S.I.; Kulek, A.; Kas-Shamoun, R.; Mika, V.; et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: Results from a pilot study. PLoS ONE 2013, 8, e80296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blodstein, P.A.; Jones, S.J.; Buechler, C.M.; Vandongen, S. Cognitive screening in mild traumatic brain injuries: Analysis of the neurobehavioral cognitive status examination when utilized during initial trauma hospitalization. J. Neurotrauma. 1997, 14, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Chamoun, R.; Suki, D.; Gopinath, S.P.; Goodman, J.C.; Robertson, C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010, 113, 564–570. [Google Scholar] [CrossRef]
- Sun, D.A.; Deshpande, L.S.; Sombati, S.; Baranova, A.; Wilson, M.S.; Hamm, R.J.; DeLorenzo, R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008, 27, 1659–1672. [Google Scholar] [CrossRef] [Green Version]
- Girouard, H.; Wang, G.; Gallo, E.F.; Anrather, J.; Zhou, P.; Pickel, V.M.; Iadecola, C. NMDA Receptor Activation Increases Free Radical Production through Nitric Oxide and NOX2. J. Neurosci. 2009, 29, 2545–2552. [Google Scholar] [CrossRef] [Green Version]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; Macdonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef]
- Lipton, S. The Molecular Basis of Memantine Action in Alzheimers Disease and Other Neurologic Disorders: Low-affinity, Uncompetitive Antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Block, F.; Schwarz, M. Memantine reduces functional and morphological consequences induced by global ischemia in rats. Neurosci. Lett. 1996, 208, 41–44. [Google Scholar] [CrossRef]
- Chen, H.-S.; Wang, Y.F.; Rayudu, P.V.; Edgecomb, P.; Neill, J.C.; Segal, M.M.; Lipton, S.A.; E Jensen, F. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience 1998, 86, 1121–1132. [Google Scholar] [CrossRef]
- Dogan, A.; Eras, M.A.; Rao, V.L.R.; Dempsey, R.J. Protective effects of memantine against ischemia-reperfusion injury in spontaneously hypertensive rats. Acta Neurochir. 1999, 141, 1107–1113. [Google Scholar] [CrossRef]
- Krieglstein, J.; El Nasr, M.S.; Lippert, K. Neuroprotection by memantine as increased by hypothermia and nimodipine. Eur. J. Pharm. Sci. 1997, 5, 71–77. [Google Scholar] [CrossRef]
- Rao, V.L.R.; Dogan, A.; Todd, K.G.; Bowen, K.K.; Dempsey, R.J. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001, 911, 96–100. [Google Scholar]
- Biegon, A.; Fry, P.A.; Paden, C.M.; Alexandrovich, A.; Tsenter, J.; Shohami, E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. USA 2004, 101, 5117–5122. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Qiu, J.; Alcon, S.; Hashi, J.; Rotenberg, A.; Sun, Y.; Meehan, W.P.; Mannix, R. Memantine improves outcomes after repetitive traumatic brain injury. Behav. Brain Res. 2018, 340, 195–204. [Google Scholar] [CrossRef]
- Effgen, G.B.; Morrison, B. Memantine reduced cell death, astrogliosis, and functional deficits in an in vitro model of repetitive mild traumatic brain injury. J. Neurotrauma. 2017, 34, 934–942. [Google Scholar] [CrossRef]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting Traumatic Brain Injury: From Molecular Mechanisms to Therapeutic Interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.C.; Collins, M.; Lovell, M.; Kontos, A.P. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J. Head Trauma Rehabil. 2013, 28, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.M.; Zafonte, R.D. Amantadine and memantine: A comprehensive review for acquired brain injury. Brain Inj. 2020, 34, 299–315. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. For the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GraphPad QuickCalcs: T-Test Calculator. Graphpad.com, 2020. Available online: https://www.graphpad.com/quickcalcs/ttest1/?Format=SD (accessed on 3 August 2020).
- Mokhtari, M.; Nayeb-Aghaei, H.; Kouchek, M.; Miri, M.M.; Goharani, R.; Amoozandeh, A.; Salamat, S.A.; Sistanizad, M. Effect of Memantine on Serum Levels of Neuron-Specific Enolase and on the Glasgow Coma Scale in Patients with Moderate Traumatic Brain Injury. J. Clin. Pharmacol. 2018, 58, 42–47. [Google Scholar] [CrossRef]
- Litvinenko, I.V.; Emelin, A.I.; Vorob’Ev, S.V.; Lobzin, V.I. Clinical features of the formation and possibilities of treatment of posttraumatic cognitive disturbances. Zh. Nevrol. Psikhiatr. Im S S Korsakova 2010, 110, 60–66. [Google Scholar]
- Rupright, S.; Johnstone, G. Effect of Namenda on Short Term Memory and Attention in Patients with Mild to Moderate Traumatic Brain Injury. ClinicalTrials.gov, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT00462228 (accessed on 26 July 2020).
- Hammond, F. Memantine for Neuroprotection and Cognitive Enhancement Following Traumatic Brain Injury. ClinicalTrials.gov, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02240589 (accessed on 26 July 2020).
- Ergün, R.; Bostanci, U.; Akdemir, G.; Beskonakli, E.; Kaptanoğlu, E.; Gürsoy, F.; Taşkin, Y. Prognostic value of serum neuron-specific enolase levels after head injury. Neurol. Res. 1998, 20, 418–420. [Google Scholar] [CrossRef]
- Ross, S.A.; Cunningham, R.T.; Johnston, C.F.; Rowlands, B.J. Neuron-specific enolase as an aid to outcome prediction in head injury. Br. J. Neurosurg. 1996, 10, 471–476. [Google Scholar] [CrossRef]
- Zurek, J.; Fedora, M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. 2012, 154, 93–103. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Hennes, H.; Gorelick, M.H.; Wells, R.G.; Walsh-Kelly, C.M. Serum neuron-specific enolase as a predictor of short-term outcome in children with closed traumatic brain injury. Acad. Emerg. Med. 2005, 12, 732–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.M.; Kufera, J.; Lindell, A.; Murdock, K.R.; Menaker, J.; Bochicchio, G.; Aarabi, B.; Scalea, T.M. Association of CSF Biomarkers and Secondary Insults Following Severe Traumatic Brain Injury. Neurocritical. Care 2011, 14, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Topolovec-Vranic, J.; Pollmann-Mudryj, M.-A.; Ouchterlony, D.; Klein, D.; Spence, J.; Romaschin, A.; Rhind, S.; Tien, H.C.; Baker, A.J. The Value of Serum Biomarkers in Prediction Models of Outcome after Mild Traumatic Brain Injury. J. Trauma Inj. Infect. Crit. Care 2011, 71, S478–S486. [Google Scholar] [CrossRef] [PubMed]
- Gradisek, P.; Osredkar, J.; Korsic, M.; Kremzar, B. Multiple indicators model of long-term mortality in traumatic brain injury. Brain Inj. 2012, 26, 1472–1481. [Google Scholar] [CrossRef]
- Chabok, S.Y.; Moghadam, A.D.; Saneei, Z.; Amlashi, F.G.; Leili, E.K.; Amiri, Z.M. Neuron-specific enolase and S100BB as outcome predictors in severe diffuse axonal injury. J. Trauma Acute Care Surg. 2012, 72, 1654–1657. [Google Scholar] [CrossRef]
- Begaz, T.; Kyriacou, D.N.; Segal, J.; Bazarian, J.J. Serum Biochemical Markers for Post-Concussion Syndrome in Patients with Mild Traumatic Brain Injury. J. Neurotrauma. 2006, 23, 1201–1210. [Google Scholar] [CrossRef]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The Prognostic Value of Serum Neuron-Specific Enolase in Traumatic Brain Injury: Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef] [Green Version]
- Johnsson, P.; Blomquist, S.; Lührs, C.; Malmkvist, G.; Alling, C.; Solem, J.-O.; Ståhl, E. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann. Thorac. Surg. 2000, 69, 750–754. [Google Scholar] [CrossRef]
- Beaudeux, J.L.; Leger, P.; Dequen, L.; Gandjbakhch, I.; Coriat, P.; Foglietti, M.J. Influence of hemolysis on the measurement of S-100beta protein and neuron-specific enolase plasma concentrations during coronary artery bypass grafting. Clin. Chem. 2000, 46, 989–990. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.W.; Liu, R.; Li, X.; Wang, Y.; Fu, Y.H.; Li, H.Y.; Yan, Y.; Gao, X.Y. High serum neuron-specific enolase level is associated with mild cognitive impairment in patients with diabetic retinopathy. Diabetes Metab. Syndr. Obes. 2020, 13, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Kok, W.F.; Koerts, J.; Tucha, O.; Scheeren, T.W.L.; Absalom, A.R. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia 2017, 72, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Borg, K.; Bonomo, J.; Jauch, E.C.; Kupchak, P.; Stanton, E.B.; Sawadsky, B. Serum levels of biochemical markers of traumatic brain injury. Int. Sch. Res. Not. 2012, 2012, 417313. [Google Scholar] [CrossRef] [Green Version]
- Jennett, B. Scale and Scope of the Problem. In Rehabilitation of the Adult and Child with Traumatic Brain Injury; Rosenthal, M., Bond, M., Griffith, E., Miller, J.F., Eds.; F. A. Davis: Philadelphia, PA, USA, 1990; pp. 3–7. [Google Scholar]
- Majumder, P.; Khandelwal, S.; Sood, M.; Nehra, A.; Sharma, B. A comparative study of cognitive function following traumatic brain injury: Significance of initial Glasgow coma scale score to predict cognitive outcome. J. Ment. Heal. Hum. Behav. 2015, 20, 59. [Google Scholar] [CrossRef]
- Rao, V.; Lyketsos, C. Neuropsychiatric Sequelae of Traumatic Brain Injury. J. Psychosom. Res. 2000, 41, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, C.G.; Danysz, W.; Quack, G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Wenk, G.L. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003, 9, 275–308. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Chen, X.-H.; Iwata, A.; Graham, D.I. Amyloid β accumulation in axons after traumatic brain injury in humans. J. Neurosurg. 2003, 98, 1072–1077. [Google Scholar] [CrossRef]
- Gentleman, S.M.; Nash, M.J.; Sweeting, C.J.; Graham, D.I.; Roberts, G.W. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993, 160, 139–144. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Abrahamson, E.E.; Ciallella, J.R.; Paljug, W.R.; Wisniewski, S.R.; Clark, R.S.; Ikonomovic, M.D. Association of increased cortical soluble Aβ42 levels with diffuse plaques after severe brain injury in humans. Arch Neurol. 2007, 64, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, H.P. On the paradox of ion channel blockade and its benefits in the treatment of Alzheimer disease. Med Hypotheses 2005, 65, 259–265. [Google Scholar] [CrossRef]

| Study | Study Year | Blinding | Time from TBI Event | Severity of TBI | Study Design | Drug Regimen | Outcome Measures |
|---|---|---|---|---|---|---|---|
| Mokhtari et al. [37] | 2017 |
| Within 24 h | Moderate TBI | RCT—parallel group | Memantine 30 mg twice daily for 7 days | Serum Neurons Specific Enolase GCS |
| Litvinenko et al. [38] | 2010 | No Mention of Blinding | >6 Months Average is 2.5 Years 95% ranged from 1.5 to 3.4 years | 73% Severe TBI 22% Moderate TBI 5% Mild TBI | RCT—parallel group Control received piracetam | Memantine titrated up to 10 mg twice daily over 4 weeks. Piracetam 2.4 g per day | Mattis Dementia Scale MMSE S-test FAB HAM-D Clock drawing |
| Rupright & Johnstone [39] | 2013 |
| More than 1 year | Mild or Moderate TBI | RCT—cluster group with cross over design Control received placebo | Titrated up to 20 mg. Duration is 12 weeks, followed by 4 weeks washout then cross over | Verbal Memory (HVLT-R TRLS, HVLT-R DRS) Visual Memory (BVMT-R TRS, BVMT-R DRS) Processing speed (TMT-A) Attention (TMT-B) Memory and Processing speed (SDMT-W, SDMT-O) |
| Hammond [40] | 2017 |
| Within 48 h | Severe TBI | RCT—parallel group Control received placebo | (Days 1 to 3 and Days 21 to 168) 10 mg twice daily (Days 3 to 21) 20 mg twice daily | Verbal Memory CVLT-II LD FR, CVLT-II T1-5 FR) Visual Memory (BVMT-R DR, BVMT-R Learning) Attention (TMT-B) Attention, cognitive flexibility and processing speed (S1) Impulse Control (BRIEF Inhibit) Anger (TBI-QOL Anger) |
| Study | Demographics and Mechanism of Injury (MOI) | Adverse Events | ||
|---|---|---|---|---|
| Therapeutic Arm | Control Arm | Therapeutic Arm | Control Arm | |
| Mokhtari et al. [37] | 22 People
| 19 People
| No mention of adverse events | No mention of adverse events |
| MOI | MOI | |||
| 3 fall1 8 motor vehicle accident | 3 fall 16 motor vehicle accident | |||
| Litvinenko et al. [38] |
|
| 1 patient developed anxiety | 2 patients developed anxiety |
| No mention of MOI | No mention of MOI | |||
| Rupright and Johnstone [39] | Cross over design |
|
| |
| ||||
| No mention of MOI | ||||
| Hammond [40] | At Initial enrolment
| At Initial enrolment
4 Males, 0 Females or 3 Males, 1 Females or 2 Males, 2 Females No mention of MOI |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Ali, A.S.; Kadir, B.; Ahmed, Z.; Di Pietro, V. Effects of Memantine in Patients with Traumatic Brain Injury: A Systematic Review. Trauma Care 2021, 1, 1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/traumas1010001
Khan S, Ali AS, Kadir B, Ahmed Z, Di Pietro V. Effects of Memantine in Patients with Traumatic Brain Injury: A Systematic Review. Trauma Care. 2021; 1(1):1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/traumas1010001
Chicago/Turabian StyleKhan, Sungeen, Ayesha S. Ali, Bryar Kadir, Zubair Ahmed, and Valentina Di Pietro. 2021. "Effects of Memantine in Patients with Traumatic Brain Injury: A Systematic Review" Trauma Care 1, no. 1: 1-14. https://0-doi-org.brum.beds.ac.uk/10.3390/traumas1010001








