Development of Multilayer Mesenchymal Stem Cell Cell Sheets
Abstract
:1. Introduction
2. Mesenchymal Stem Cells Therapies
2.1. Injection of Single Cells
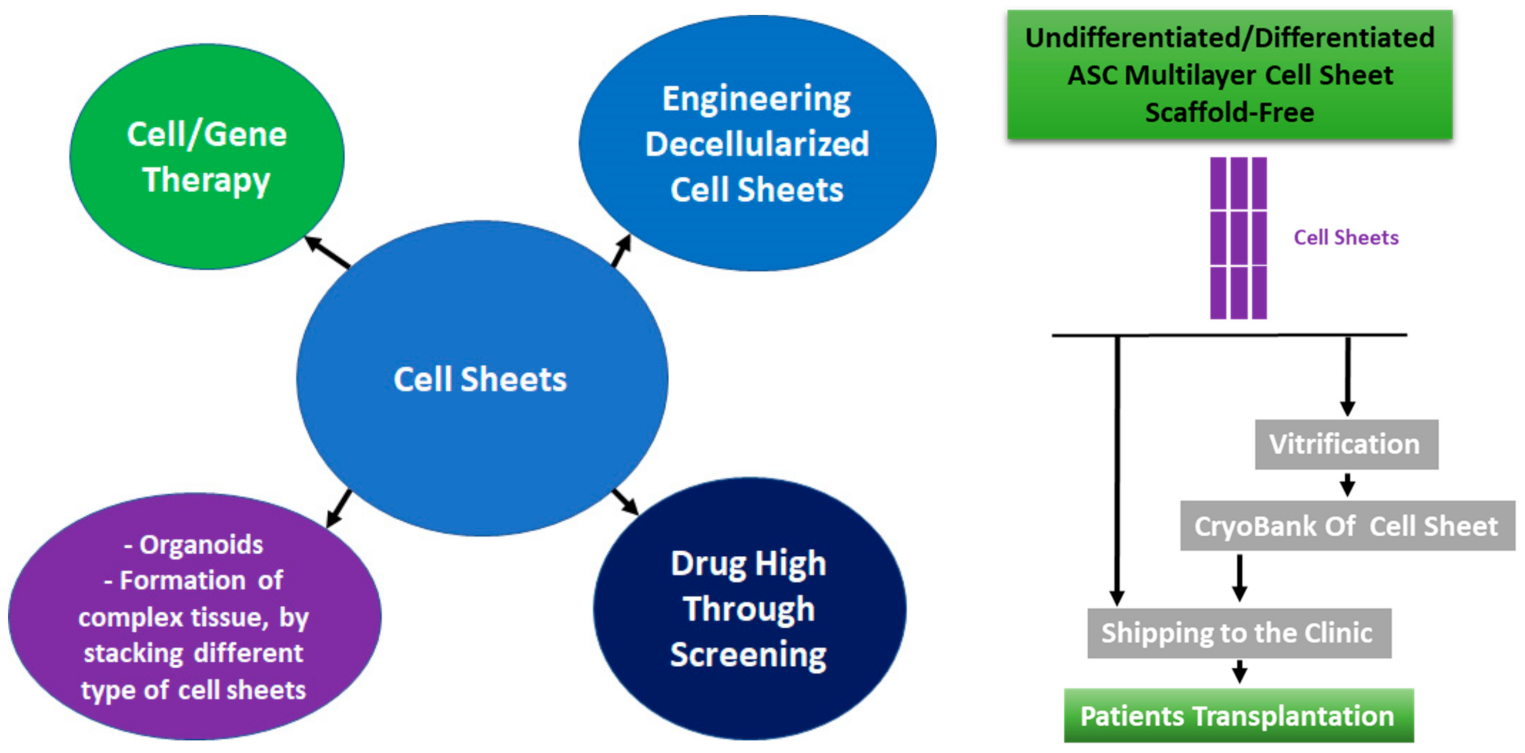
2.2. Cell Sheet Technology
2.3. Biodistribution
3. Cryopreservation of the Cell Sheets
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimlin, L.; Kassis, J.; Virador, V. 3D in vitro tissue models and their potential for drug screening. Expert Opin. Drug Discov. 2013, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilic, D.; Ogilvie, C. Concise Review: Human Embryonic Stem Cells-What Have We Done? What Are We Doing? Where Are We Going? Stem Cells 2017, 35, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, S.; Mahdizadeh, H.; Saric, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B.T. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.L. Some Ethical Concerns About Human Induced Pluripotent Stem Cells. Sci. Eng. Ethics 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Zacharias, D.G.; Nelson, T.J.; Mueller, P.S.; Hook, C.C. The science and ethics of induced pluripotency: What will become of embryonic stem cells? Mayo Clin. Proc. 2011, 86, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef]
- Yu, T.T.L.; Gupta, P.; Ronfard, V.; Vertes, A.A.; Bayon, Y. Recent Progress in European Advanced Therapy Medicinal Products and Beyond. Front. Bioeng. Biotechnol. 2018, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Zomer, H.D.; Vidane, A.S.; Goncalves, N.N.; Ambrosio, C.E. Mesenchymal and induced pluripotent stem cells: General insights and clinical perspectives. Stem Cells Cloning 2015, 8, 125–134. [Google Scholar] [CrossRef]
- Miana, V.V.; Gonzalez, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert Opin. Biol. Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [Green Version]
- Hatina, J.; Kripnerova, M.; Houfkova, K.; Pesta, M.; Kuncova, J.; Sana, J.; Slaby, O.; Rodriguez, R. Sarcoma Stem Cell Heterogeneity. Adv. Exp. Med. Biol. 2019, 1123, 95–118. [Google Scholar]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R.C.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS ONE 2011, 6, e20526. [Google Scholar] [CrossRef] [Green Version]
- Sathananthan, H.; Selvaraj, K.; Clark, J. The fine structure of human germ layers in vivo: Clues to the early differentiation of embryonic stem cells in vitro. Reprod. Biomed. Online 2011, 23, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R. Immunomodulatory Properties of Mesenchymal Stromal Cells: Still Unresolved “Yin and Yang”. Curr. Stem Cell Res. Ther. 2019, 14, 344–350. [Google Scholar] [CrossRef]
- Kolb, H.J. Hematopoietic stem cell transplantation and cellular therapy. HLA 2017, 89, 267–277. [Google Scholar] [CrossRef]
- Hao, M.; Wang, R.; Wang, W. Cell Therapies in Cardiomyopathy: Current Status of Clinical Trials. Anal. Cell Pathol. 2017, 2017, 9404057. [Google Scholar] [CrossRef] [Green Version]
- Duelen, R.; Sampaolesi, M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, U.G.; Vanikar, A.V.; Trivedi, H.L. Stem cell therapy: An emerging modality in glomerular diseases. Cytotherapy 2017, 19, 333–348. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Afshari, J.T.; Keramati, M.R.; Alamdari, D.H.; Ganjibakhsh, M.; Zarmehri, A.M.; Jangjoo, A.; Sadeghian, M.H.; Ameri, M.A.; Moinzadeh, L. Differentiation of adipocytes and osteocytes from human adipose and placental mesenchymal stem cells. Iran J. Basic Med. Sci. 2015, 18, 259–266. [Google Scholar]
- Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy 2011, 13, 310–317. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Mardani, M.; Esfandiari, E.; Salehi, H.; Zarkesh Esfahani, S.H. Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Mol. Biotechnol. 2014, 56, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.M.; Perez Cano, R.; Castro, Y.; Arenas, L.; Garcia, J.; Fernandez-Santos, M.E. Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, e189–e197. [Google Scholar] [CrossRef] [PubMed]
- Jun Hong, S.; Rogers, P.I.; Kihlken, J.; Warfel, J.; Bull, C.; Deuter-Reinhard, M.; Feng, D.; Xie, J.; Kyle, A.; Merfeld-Clauss, S.; et al. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter. Cardiovasc. Interv. 2015, 86, E38–E48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelatti, M.V.; Gomes, J.P.; Vieira, N.M.; Cangussu, E.; Landini, V.; Andrade, T.; Sartori, M.; Petrus, L.; Zatz, M. Transplantation of Human Adipose Mesenchymal Stem Cells in Non-Immunosuppressed GRMD Dogs is a Safe Procedure. Stem Cell Rev. Rep. 2016, 12, 448–453. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, G.; Lue, T.F. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012, 21, 2770–2778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Asano, K.; Yoshimura, S.; Nakane, A. Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Staphylococcal Enterotoxin A-Induced Toxic Shock. Infect. Immun. 2015, 83, 3490–3496. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.J.; Traktuev, D.O.; March, K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr. Opin. Organ Transplant. 2010, 15, 86–91. [Google Scholar] [CrossRef]
- Ricco, S.; Renzi, S.; Del Bue, M.; Conti, V.; Merli, E.; Ramoni, R.; Lucarelli, E.; Gnudi, G.; Ferrari, M.; Grolli, S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int. J. Immunopathol. Pharmacol. 2013, 26 (Suppl. 1), 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Yan, H.; Fu, S.; Qian, Y.; Wang, D.; Wang, C. Allogeneic adipose-derived stem cells regenerate bone in a critical-sized ulna segmental defect. Exp. Biol. Med. 2016, 241, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Garcia-Arranz, M.; Herreros, M.D.; Gonzalez-Gomez, C.; de la Quintana, P.; Guadalajara, H.; Georgiev-Hristov, T.; Trebol, J.; Garcia-Olmo, D. Treatment of Crohn’s-Related Rectovaginal Fistula With Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. Stem Cells Transl. Med. 2016, 5, 1441–1446. [Google Scholar] [CrossRef]
- Park, K.J.; Ryoo, S.B.; Kim, J.S.; Kim, T.I.; Baik, S.H.; Kim, H.J.; Lee, K.Y.; Kim, M.; Kim, W.H. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: A pilot clinical trial. Colorectal Dis. 2016, 18, 468–476. [Google Scholar] [CrossRef]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.; Wasser, M.N.; Dijkstra, G.; van der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.J.; et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927.e6. [Google Scholar] [CrossRef] [Green Version]
- De la Portilla, F.; Alba, F.; Garcia-Olmo, D.; Herrerias, J.M.; Gonzalez, F.X.; Galindo, A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int. J. Colorectal Dis. 2013, 28, 313–323. [Google Scholar] [CrossRef]
- Park, E.J.; Kang, J.; Baik, S.H. Treatment of faecal incontinence using allogeneic-adipose-derived mesenchymal stem cells: A study protocol for a pilot randomised controlled trial. BMJ Open 2016, 6, e010450. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef]
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Hernigou, P.; Homma, Y.; Flouzat Lachaniette, C.H.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int. Orthop. 2013, 37, 2279–2287. [Google Scholar] [CrossRef]
- Wu, C.; Laswell, S.; Mentz, J.A.; Morales, R. Vibration Exposure Safety Guidelines for Surgeons Using Power-Assisted Liposuction (PAL). Aesthet. Surg. J. 2020. [Google Scholar] [CrossRef]
- Qu, Y.; Luan, J.; Mu, D.; Wang, Q.; Li, Z.; Liu, T.; Fu, S. Does Water-Jet Force Affect Cryopreserved Adipose-Derived Stem Cells? Evidence of Improved Cell Viability and Fat Graft Survival. Ann. Plast. Surg. 2020. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Sachs, P.C.; Francis, M.P.; Zhao, M.; Brumelle, J.; Rao, R.R.; Elmore, L.W.; Holt, S.E. Defining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sites. Cell Tissue Res. 2012, 349, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casteilla, L.; Planat-Benard, V.; Laharrague, P.; Cousin, B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J. Stem Cells 2011, 3, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, X.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [Green Version]
- Filley, A.C.; Henriquez, M.; Dey, M. CART Immunotherapy: Development, Success, and Translation to Malignant Gliomas and Other Solid Tumors. Front. Oncol. 2018, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Tomita, S.; Li, R.K.; Weisel, R.D.; Mickle, D.A.; Kim, E.J.; Sakai, T.; Jia, Z.Q. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999, 100, II-247–II-256. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, B.D.S.; Almeida, F.M.; Sales, C.M.; de Lima, S.; Martinez, A.M.B. Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice. Neural Regen. Res. 2018, 13, 1046–1053. [Google Scholar]
- Lykhmus, O.; Koval, L.; Voytenko, L.; Uspenska, K.; Komisarenko, S.; Deryabina, O.; Shuvalova, N.; Kordium, V.; Ustymenko, A.; Kyryk, V.; et al. Intravenously Injected Mesenchymal Stem Cells Penetrate the Brain and Treat Inflammation-Induced Brain Damage and Memory Impairment in Mice. Front. Pharmacol. 2019, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.U.; Yoon, Y.; Choi, K.U.; Jo, K.R.; Kim, N.; Lee, E.; Kim, W.H.; Kweon, O.K. Therapeutic Effects of Intravenous Injection of Fresh and Frozen Thawed HO-1-Overexpressed Ad-MSCs in Dogs with Acute Spinal Cord Injury. Stem Cells Int. 2019, 2019, 8537541. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.; Wang, W.; Liang, L.; Han, Z.B.; Li, Z.; Geng, J.; Zhao, M.; Jia, H.; Feng, J.; Wei, Z.; et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther. 2018, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [Green Version]
- Spichal, M.; Fabre, E. The Emerging Role of the Cytoskeleton in Chromosome Dynamics. Front. Genet. 2017, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Viita, T.; Vartiainen, M.K. From Cytoskeleton to Gene Expression: Actin in the Nucleus. Handb. Exp. Pharmacol. 2017, 235, 311–329. [Google Scholar]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kwon, Y.R.; Kim, H.J.; Lee, S.; Kim, Y.J. Enhanced differentiation of mesenchymal stromal cells by three-dimensional culture and azacitidine. Blood Res. 2017, 52, 18–24. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylostalo, J.H. Mesenchymal Stem Cell (MSC) Aggregate Formation in vivo. Bio-Protocol 2014, 4, e1181. [Google Scholar] [CrossRef] [Green Version]
- Oeller, M.; Laner-Plamberger, S.; Hochmann, S.; Ketterl, N.; Feichtner, M.; Brachtl, G.; Hochreiter, A.; Scharler, C.; Bieler, L.; Romanelli, P.; et al. Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics 2018, 8, 1421–1434. [Google Scholar] [CrossRef] [Green Version]
- Perlee, D.; de Vos, A.F.; Scicluna, B.P.; Maag, A.; Mancheno, P.; de la Rosa, O.; Dalemans, W.; Florquin, S.; Van’t Veer, C.; Lombardo, E.; et al. Role of tissue factor in the procoagulant and antibacterial effects of human adipose-derived mesenchymal stem cells during pneumosepsis in mice. Stem Cell Res. Ther. 2019, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef]
- Coppin, L.C.F.; Smets, F.; Ambroise, J.; Sokal, E.E.M.; Stephenne, X. Infusion-related thrombogenesis by liver-derived mesenchymal stem cells controlled by anticoagulant drugs in 11 patients with liver-based metabolic disorders. Stem Cell Res. Ther. 2020, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [Green Version]
- Fabian, C.; Naaldijk, Y.; Leovsky, C.; Johnson, A.A.; Rudolph, L.; Jaeger, C.; Arnold, K.; Stolzing, A. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res. Ther. 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyongyosi, M.; Blanco, J.; Marian, T.; Tron, L.; Petnehazy, O.; Petrasi, Z.; Hemetsberger, R.; Rodriguez, J.; Font, G.; Pavo, I.J.; et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ. Cardiovasc. Imaging 2008, 1, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Le Maitre, C.L.; Baird, P.; Freemont, A.J.; Hoyland, J.A. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res. Ther. 2009, 11, R20. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Wang, X. Biodegradable Polymers and Stem Cells for Bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Markomol. Chem. 1990, 11, 571–576. [Google Scholar]
- Ohki, T.; Yamamoto, M. Esophageal regenerative therapy using cell sheet technology. Regen. Ther. 2020, 13, 8–17. [Google Scholar] [CrossRef]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Delarue, C.; Contesse, V.; Lenglet, S.; Sicard, F.; Perraudin, V.; Lefebvre, H.; Kodjo, M.; Leboulenger, F.; Yon, L.; Gallo-Payet, N.; et al. Role of neurotransmitters and neuropeptides in the regulation of the adrenal cortex. Rev. Endocr. Metab. Disord. 2001, 2, 253–267. [Google Scholar] [CrossRef]
- Yasuda, K. Dominant rule of community effect in synchronized beating behavior of cardiomyocyte networks. Biophys. Rev. 2020, 12, 481–501. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, W.; Wang, J.; Yang, G.; Yin, S.; Tang, T.; Yu, C.; Jiang, X. Recent advances in cell sheet technology for bone and cartilage regeneration: From preparation to application. Int. J. Oral Sci. 2019, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Engineering, differentiation and harvesting of human adipose-derived stem cell multilayer cell sheets. Regen. Med. 2019, 14, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Raposio, E. From liposuction to adipose-derived stem cells: Indications and technique. Acta Biomed. 2019, 90, 197–208. [Google Scholar]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, R.A.; Aronowitz, J.A.; Dos-Anjos Vilaboa, S. Use of Freshly Isolated Human Adipose Stromal Cells for Clinical Applications. Aesthet. Surg. J. 2017, 37, S4–S8. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.R.; Orellana, M.D.; Mizukami, A.; Fernandes, T.R.; Fontes, A.M.; Suazo, C.A.; Oliveira, V.C.; Covas, D.T.; Swiech, K. Growth and functional harvesting of human mesenchymal stromal cells cultured on a microcarrier-based system. Biotechnol. Prog. 2014, 30, 889–895. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010, 5, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Yamato, M.; Mitani, G.; Takagaki, T.; Hamahashi, K.; Nakamura, Y.; Ishihara, M.; Matoba, R.; Kobayashi, H.; Okano, T.; et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e003918. [Google Scholar] [CrossRef]
- Devito, L.; Petrova, A.; Miere, C.; Codognotto, S.; Blakely, N.; Lovatt, A.; Ogilvie, C.; Khalaf, Y.; Ilic, D. Cost-effective master cell bank validation of multiple clinical-grade human pluripotent stem cell lines from a single donor. Stem Cells Transl. Med. 2014, 3, 1116–1124. [Google Scholar] [CrossRef]
- Shan, X.; Hu, D. Bone engineering by cell sheet technology to repair mandibular defects. Exp. Ther. Med. 2017, 14, 5007–5011. [Google Scholar] [CrossRef] [Green Version]
- Assuncao, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Spater, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010, 267, 54–70. [Google Scholar] [CrossRef]
- Nakao, M.; Kim, K.; Nagase, K.; Grainger, D.W.; Kanazawa, H.; Okano, T. Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: Structural characteristics of MSC sheets. Stem Cell Res. Ther. 2019, 10, 353. [Google Scholar] [CrossRef]
- Kim, S.R.; Yi, H.J.; Lee, Y.N.; Park, J.Y.; Hoffman, R.M.; Okano, T.; Shim, I.K.; Kim, S.C. Engineered mesenchymal stem-cell-sheets patches prevents postoperative pancreatic leakage in a rat model. Sci. Rep. 2018, 8, 360. [Google Scholar] [CrossRef] [Green Version]
- Doogue, M.P.; Polasek, T.M. The ABCD of clinical pharmacokinetics. Ther. Adv. Drug Saf. 2013, 4, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Lauridsen, H.; Foldager, C.B.; Hansen, L.; Pedersen, M. Non-invasive cell tracking of SPIO labeled cells in an intrinsic regenerative environment: The axolotl limb. Exp. Ther. Med. 2018, 15, 3311–3319. [Google Scholar] [CrossRef]
- Wu, L.; Liu, F.; Liu, S.; Xu, X.; Liu, Z.; Sun, X. Perfluorocarbons-Based (19)F Magnetic Resonance Imaging in Biomedicine. Int. J. Nanomed. 2020, 15, 7377–7395. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Bardag-Gorce, F.; Wood, A.; Sota, H.; Niihara, Y. Direct labeling of 19F-perfluorocarbon onto multilayered cell sheet for MRI-based non-invasive cell tracking. Tissue Eng. Regen. Med. 2015, 12, 371–378. [Google Scholar] [CrossRef]
- Oliva, J.; Bardag-Gorce, F.; Niihara, Y. Clinical Trials of Limbal Stem Cell Deficiency Treated with Oral Mucosal Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Sotozono, C.; Bentley, A.J.; Mano, S.; Inatomi, T.; Koizumi, N.; Fullwood, N.J.; Kinoshita, S. Long-term phenotypic study after allogeneic cultivated corneal limbal epithelial transplantation for severe ocular surface diseases. Ophthalmology 2010, 117, 2247–2254.e1. [Google Scholar] [CrossRef]
- Meek, S.; Wei, J.; Oh, T.; Watson, T.; Olavarrieta, J.; Sutherland, L.; Carlson, D.F.; Salzano, A.; Chandra, T.; Joshi, A.; et al. A Stem Cell Reporter for Investigating Pluripotency and Self-Renewal in the Rat. Stem Cell Rep. 2020, 14, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Jurgielewicz, P.; Harmsen, S.; Wei, E.; Bachmann, M.H.; Ting, R.; Aras, O. New imaging probes to track cell fate: Reporter genes in stem cell research. Cell Mol. Life Sci. 2017, 74, 4455–4469. [Google Scholar] [CrossRef]
- Chalfie, M. GFP: Lighting up life. Proc. Natl. Acad. Sci. USA 2009, 106, 10073–10080. [Google Scholar] [CrossRef]
- Soghomonyan, S.; Hajitou, A.; Rangel, R.; Trepel, M.; Pasqualini, R.; Arap, W.; Gelovani, J.G.; Alauddin, M.M. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat. Protoc. 2007, 2, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Iwasawa, C.; Tamura, R.; Sugiura, Y.; Suzuki, S.; Kuzumaki, N.; Narita, M.; Suematsu, M.; Nakamura, M.; Yoshida, K.; Toda, M.; et al. Increased Cytotoxicity of Herpes Simplex Virus Thymidine Kinase Expression in Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2019, 20, 810. [Google Scholar] [CrossRef] [Green Version]
- Deans, A.E.; Wadghiri, Y.Z.; Bernas, L.M.; Yu, X.; Rutt, B.K.; Turnbull, D.H. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn. Reson. Med. 2006, 56, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Brissot, P.; Troadec, M.B.; Loreal, O.; Brissot, E. Pathophysiology and classification of iron overload diseases; update 2018. Transfus. Clin. Biol. 2019, 26, 80–88. [Google Scholar] [CrossRef]
- Gherase, M.R.; Fleming, D.E.B. Probing Trace Elements in Human Tissues with Synchrotron Radiation. Crystals 2020, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Ozawa, T. Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission. Int. J. Mol. Sci. 2020, 21, 6538. [Google Scholar] [CrossRef]
- Perrin, J.; Capitao, M.; Mougin-Degraef, M.; Guerard, F.; Faivre-Chauvet, A.; Rbah-Vidal, L.; Gaschet, J.; Guilloux, Y.; Kraeber-Bodere, F.; Cherel, M.; et al. Cell Tracking in Cancer Immunotherapy. Front. Med. 2020, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Jokerst, J.V. Stem Cell Tracking with Nanoparticle-Based Ultrasound Contrast Agents. Methods Mol. Biol. 2020, 2126, 141–153. [Google Scholar]
- Kubelick, K.P.; Snider, E.J.; Ethier, C.R.; Emelianov, S. Development of a stem cell tracking platform for ophthalmic applications using ultrasound and photoacoustic imaging. Theranostics 2019, 9, 3812–3824. [Google Scholar] [CrossRef]
- Vandergaast, R.; Khongwichit, S.; Jiang, H.; DeGrado, T.R.; Peng, K.W.; Smith, D.R.; Russell, S.J.; Suksanpaisan, L. Enhanced noninvasive imaging of oncology models using the NIS reporter gene and bioluminescence imaging. Cancer Gene Ther. 2020, 27, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis. 2018, 4, 11. [Google Scholar] [CrossRef]
- MacAskill, M.G.; Tavares, A.S.; Wu, J.; Lucatelli, C.; Mountford, J.C.; Baker, A.H.; Newby, D.E.; Hadoke, P.W. PET Cell Tracking Using (18)F-FLT is Not Limited by Local Reuptake of Free Radiotracer. Sci. Rep. 2017, 7, 44233. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.W.; Choi, S.I.; Lee, S.J.; Oh, Y.T.; Park, G.; Park, N.Y.; Yoon, K.A.; Kim, S.; Kim, D.; Kim, Y.H.; et al. The Effectiveness of Ferritin as a Contrast Agent for Cell Tracking MRI in Mouse Cancer Models. Yonsei Med. J. 2017, 58, 51–58. [Google Scholar] [CrossRef]
- Ansari, A.M.; Ahmed, A.K.; Matsangos, A.E.; Lay, F.; Born, L.J.; Marti, G.; Harmon, J.W.; Sun, Z. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev. Rep. 2016, 12, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, A.M.; Kurecka, P.; Yim, T.K.; Kozemchak, C.; Deb, A.; Dostal, L.; Sun, C.J.; Brewe, D.L.; Barrea, R.; Penner-Hahn, J.E. Development of a single-cell X-ray fluorescence flow cytometer. J. Synchrotron Radiat. 2016, 23 Pt 4, 901–908. [Google Scholar] [CrossRef]
- Wang, Z.G.; Liu, S.L.; Hu, Y.J.; Tian, Z.Q.; Hu, B.; Zhang, Z.L.; Pang, D.W. Dissecting the Factors Affecting the Fluorescence Stability of Quantum Dots in Live Cells. ACS Appl. Mater. Interfaces 2016, 8, 8401–8408. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, A.; Jiang, P.; Yang, M.; Yamamoto, N.; Moriwaki, H.; Saji, S.; Hoffman, R.M. The Use of Living Cancer Cells Expressing Green Fluorescent Protein in the Nucleus and Red Fluorescence Protein in the Cytoplasm for Real-time Confocal Imaging of Chromosome and Cytoplasmic Dynamics During Mitosis. Anticancer Res. 2015, 35, 2553–2557. [Google Scholar] [PubMed]
- Kim, J.E.; Kalimuthu, S.; Ahn, B.C. In vivo cell tracking with bioluminescence imaging. Nucl. Med. Mol. Imaging 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushie, M.J.; Pickering, I.J.; Korbas, M.; Hackett, M.J.; George, G.N. Elemental and chemically specific X-ray fluorescence imaging of biological systems. Chem. Rev. 2014, 114, 8499–8541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penheiter, A.R.; Russell, S.J.; Carlson, S.K. The sodium iodide symporter (NIS) as an imaging reporter for gene, viral, and cell-based therapies. Curr. Gene Ther. 2012, 12, 33–47. [Google Scholar] [CrossRef]
- Gildehaus, F.J.; Haasters, F.; Drosse, I.; Wagner, E.; Zach, C.; Mutschler, W.; Cumming, P.; Bartenstein, P.; Schieker, M. Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Mol. Imaging Biol. 2011, 13, 1204–1214. [Google Scholar] [CrossRef]
- Walling, M.A.; Novak, J.A.; Shepard, J.R. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef] [Green Version]
- Kummer, C.; Winkeler, A.; Dittmar, C.; Bauer, B.; Rueger, M.A.; Rueckriem, B.; Heneka, M.T.; Vollmar, S.; Wienhard, K.; Fraefel, C.; et al. Multitracer positron emission tomographic imaging of exogenous gene expression mediated by a universal herpes simplex virus 1 amplicon vector. Mol. Imaging 2007, 6, 181–192. [Google Scholar] [CrossRef]
- Hackman, T.; Doubrovin, M.; Balatoni, J.; Beresten, T.; Ponomarev, V.; Beattie, B.; Finn, R.; Bornmann, W.; Blasberg, R.; Tjuvajev, J.G. Imaging expression of cytosine deaminase-herpes virus thymidine kinase fusion gene (CD/TK) expression with [124I]FIAU and PET. Mol. Imaging 2002, 1, 36–42. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.C. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev. Rep. 2012, 8, 243–250. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonne, A.; Delorme, B.; Herault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Sordi, V.; Malosio, M.L.; Marchesi, F.; Mercalli, A.; Melzi, R.; Giordano, T.; Belmonte, N.; Ferrari, G.; Leone, B.E.; Bertuzzi, F.; et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005, 106, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Hocking, A.M. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care 2015, 4, 623–630. [Google Scholar] [CrossRef]
- Lo, C.Y.; Antonopoulos, A.; Dell, A.; Haslam, S.M.; Lee, T.; Neelamegham, S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials 2013, 34, 8213–8222. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Li, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; et al. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef] [Green Version]
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: Knowns and unknowns. Intensive Care Med. Exp. 2019, 7, 41. [Google Scholar] [CrossRef]
- Nystedt, J.; Anderson, H.; Tikkanen, J.; Pietila, M.; Hirvonen, T.; Takalo, R.; Heiskanen, A.; Satomaa, T.; Natunen, S.; Lehtonen, S.; et al. Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells 2013, 31, 317–326. [Google Scholar] [CrossRef]
- Valles, G.; Bensiamar, F.; Maestro-Paramio, L.; Garcia-Rey, E.; Vilaboa, N.; Saldana, L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.E.; Yoo, D.; LeRoux, M.A.; Danilkovitch-Miagkova, A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm. Allergy Drug Targets 2009, 8, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Klinker, M.W.; Wei, C.H. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J. Stem Cells 2015, 7, 556–567. [Google Scholar] [CrossRef]
- Monguio-Tortajada, M.; Bayes-Genis, A.; Rosell, A.; Roura, S. Are mesenchymal stem cells and derived extracellular vesicles valuable to halt the COVID-19 inflammatory cascade? Current evidence and future perspectives. Thorax 2020, 76, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.J.; Harman, R.J.; Bunnell, B.A.; Schreiber, M.A.; Xiang, C.; Wang, F.S.; Santidrian, A.F.; Minev, B.R. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J. Transl. Med. 2020, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.S.; Khan, M.A. Mesenchymal Stem Cells: A new front emerge in COVID19 treatment: Mesenchymal Stem Cells therapy for SARS-CoV2 viral infection. Cytotherapy 2020. [Google Scholar] [CrossRef]
- Hos, D.; Saban, D.R.; Bock, F.; Regenfuss, B.; Onderka, J.; Masli, S.; Cursiefen, C. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch. Ophthalmol. 2011, 129, 445–452. [Google Scholar] [CrossRef]
- Drela, K.; Stanaszek, L.; Nowakowski, A.; Kuczynska, Z.; Lukomska, B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. Stem Cells Int. 2019, 2019, 7012692. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Dominguez, X.; Vera-Donoso, C.D.; Jimenez-Trigos, E.; Vicente, J.S.; Marco-Jimenez, F. First Steps Towards Organ Banks: Vitrification of Renal Primordial. CryoLetters 2016, 37, 47–52. [Google Scholar]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.F.; Dias, A.F.; Reis, R.L.; Gomes, M.E. Cryopreservation of cell/scaffold tissue-engineered constructs. Tissue Eng. Part C Methods 2012, 18, 852–858. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered Scaffolds for Stem Cell Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1107, 73–89. [Google Scholar]
- Agmon, G.; Christman, K.L. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci. 2016, 20, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Kikuchi, A.; Aoyagi, T.; Okano, T. Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mater. Res. A 2019, 107, 955–967. [Google Scholar] [CrossRef]
- Makar, R.S.; Toth, T.L. The evaluation of infertility. Am. J. Clin. Pathol. 2002, 117 (Suppl. 1), S95–S103. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Vutyavanich, T.; Lattiwongsakorn, W.; Piromlertamorn, W.; Samchimchom, S. Repeated vitrification/warming of human sperm gives better results than repeated slow programmable freezing. Asian J. Androl. 2012, 14, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef] [Green Version]
- Asghar, W.; El Assal, R.; Shafiee, H.; Anchan, R.M.; Demirci, U. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnol. J. 2014, 9, 895–903. [Google Scholar] [CrossRef] [Green Version]
- De Vries, R.J.; Yarmush, M.; Uygun, K. Systems engineering the organ preservation process for transplantation. Curr. Opin. Biotechnol. 2019, 58, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Zaun, D.; Rosendale, J.D.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2012 Annual Data Report: Deceased organ donation. Am. J. Transplant. 2014, 14 (Suppl. 1), 167–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouna, G.M. Organ shortage crisis: Problems and possible solutions. Transplant. Proc. 2008, 40, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Theruvath, A.J.; Ge, X.; Floerchinger, B.; Jurisch, A.; Garcia-Cardena, G.; Tullius, S.G. Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives. Transpl. Int. 2010, 23, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mantecchini, L.; Paganelli, F.; Morabito, V.; Ricci, A.; Peritore, D.; Trapani, S.; Montemurro, A.; Rizzo, A.; Del Sordo, E.; Gaeta, A.; et al. Transportation of Organs by Air: Safety, Quality, and Sustainability Criteria. Transplant. Proc. 2016, 48, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Karow, A.M., Jr.; Webb, W.R. Cardiac storage with glycerol at zero centigrade. Arch. Surg. 1961, 83, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Van den Broecke, R.; Liu, J.; Van der Elst, J.; Dhont, M. Timing of FSH-stimulation and follicular development in cryopreserved human ovarian grafts. Reprod. Biomed. Online 2002, 4, 21–26. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiol. Res. Pract. 2011, 2011, 845170. [Google Scholar] [CrossRef] [Green Version]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Hayashi, A.; Maehara, M.; Uchikura, A.; Matsunari, H.; Matsumura, K.; Hyon, S.H.; Sato, M.; Nagashima, H. Development of an efficient vitrification method for chondrocyte sheets for clinical application. Regen Ther. 2020, 14, 215–221. [Google Scholar] [CrossRef]
- Ohkawara, H.; Miyagawa, S.; Fukushima, S.; Yajima, S.; Saito, A.; Nagashima, H.; Sawa, Y. Development of a vitrification method for preserving human myoblast cell sheets for myocardial regeneration therapy. BMC Biotechnol. 2018, 18, 56. [Google Scholar] [CrossRef] [Green Version]
- Tani, Y.; Sato, M.; Maehara, M.; Nagashima, H.; Yokoyama, M.; Yokoyama, M.; Yamato, M.; Okano, T.; Mochida, J. The effects of using vitrified chondrocyte sheets on pain alleviation and articular cartilage repair. J. Tissue Eng. Regen. Med. 2017, 11, 3437–3444. [Google Scholar] [CrossRef]
- Maehara, M.; Sato, M.; Watanabe, M.; Matsunari, H.; Kokubo, M.; Kanai, T.; Sato, M.; Matsumura, K.; Hyon, S.H.; Yokoyama, M.; et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Oie, Y.; Nozaki, T.; Takayanagi, H.; Hara, S.; Hayashi, R.; Takeda, S.; Mori, K.; Moriya, N.; Soma, T.; Tsujikawa, M.; et al. Development of a cell sheet transportation technique for regenerative medicine. Tissue Eng. Part C Methods 2014, 20, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Larman, M.G.; Katz-Jaffe, M.G.; McCallie, B.; Filipovits, J.A.; Gardner, D.K. Analysis of global gene expression following mouse blastocyst cryopreservation. Hum. Reprod. 2011, 26, 2672–2680. [Google Scholar] [CrossRef] [Green Version]
- Brair, V.L.; Maia, A.; Correia, L.F.L.; Barbosa, N.O.; Santos, J.D.R.; Brandao, F.Z.; Fonseca, J.F.; Batista, R.; Souza-Fabjan, J.M.G. Gene expression patterns of in vivo-derived sheep blastocysts is more affected by vitrification than slow freezing technique. Cryobiology 2020, 95, 110–115. [Google Scholar] [CrossRef]
- Bera, M.; Sengupta, K. Nuclear filaments: Role in chromosomal positioning and gene expression. Nucleus 2020, 11, 99–110. [Google Scholar] [CrossRef]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef] [Green Version]
- Golan, M.; Pribyl, J.; Pesl, M.; Jelinkova, S.; Acimovic, I.; Jaros, J.; Rotrekl, V.; Falk, M.; Sefc, L.; Skladal, P.; et al. Cryopreserved Cells Regeneration Monitored by Atomic Force Microscopy and Correlated With State of Cytoskeleton and Nuclear Membrane. IEEE Trans. Nanobiosci. 2018, 17, 485–497. [Google Scholar] [CrossRef]
- Aurich, C.; Schreiner, B.; Ille, N.; Alvarenga, M.; Scarlet, D. Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology 2016, 86, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Kopeika, J.; Thornhill, A.; Khalaf, Y. The effect of cryopreservation on the genome of gametes and embryos: Principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update 2015, 21, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- De Leeuw, F.E.; Chen, H.C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef]
- Tao, Y.; Sanger, E.; Saewu, A.; Leveille, M.C. Human sperm vitrification: The state of the art. Reprod. Biol. Endocrinol. 2020, 18, 17. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar]
- Bartolac, L.K.; Lowe, J.L.; Koustas, G.; Grupen, C.G.; Sjoblom, C. Effect of different penetrating and non-penetrating cryoprotectants and media temperature on the cryosurvival of vitrified in vitro produced porcine blastocysts. Anim. Sci. J. 2018, 89, 1230–1239. [Google Scholar] [CrossRef]
- Kummerfeld, D.M.; Raabe, C.A.; Brosius, J.; Mo, D.; Skryabin, B.V.; Rozhdestvensky, T.S. A Comprehensive Review of Genetically Engineered Mouse Models for Prader-Willi Syndrome Research. Int. J. Mol. Sci. 2021, 22, 3613. [Google Scholar] [CrossRef] [PubMed]
- Madaan, M.; Mendez, M.D. Angelman Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wang, K.H.; Kupa, J.; Duffy, K.A.; Kalish, J.M. Diagnosis and Management of Beckwith-Wiedemann Syndrome. Front. Pediatr. 2019, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Adamek, A. Insulin-Like Growth Factor 2 (IGF2) Signaling in Colorectal Cancer-From Basic Research to Potential Clinical Applications. Int. J. Mol. Sci. 2019, 20, 4915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anvar, Z.; Acurzio, B.; Roma, J.; Cerrato, F.; Verde, G. Origins of DNA methylation defects in Wilms tumors. Cancer Lett. 2019, 457, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Vitrification and storage of oral mucosa epithelial cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Bian, Y.L.; Schreurs, N.; Zhang, X.G.; Raza, S.H.A.; Fang, Q.; Wang, L.Q.; Hu, J.H. Effects of five cryoprotectants on proliferation and differentiation-related gene expression of frozen-thawed bovine calf testicular tissue. Reprod. Domest. Anim. 2018, 53, 1211–1218. [Google Scholar] [CrossRef]
- Jahan, S.; Kaushal, R.; Pasha, R.; Pineault, N. Current and Future Perspectives for the Cryopreservation of Cord Blood Stem Cells. Transfus. Med. Rev. 2021. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B. Principles of Ice-Free Cryopreservation by Vitrification. Methods Mol. Biol. 2021, 2180, 27–97. [Google Scholar]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Molecular Physiology of Freeze Tolerance in Vertebrates. Physiol. Rev. 2017, 97, 623–665. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Liu, M.; Sui, X.; Zhu, Y.; Wen, C.; Zhang, L. Advanced Biotechnology for Cell Cryopreservation. Trans. Tianjin Univ. 2020, 26, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Kong, M.J.; Jang, H.J.; Cho, J.I.; Park, E.J.; Lee, I.K.; Frokiaer, J.; Norregaard, R.; Park, K.M.; Kwon, T.H. A nonbiodegradable scaffold-free cell sheet of genome-engineered mesenchymal stem cells inhibits development of acute kidney injury. Kidney Int. 2021, 99, 117–133. [Google Scholar] [CrossRef]
- Galvez, V.; Chacon-Solano, E.; Bonafont, J.; Mencia, A.; Di, W.L.; Murillas, R.; Llames, S.; Vicente, A.; Del Rio, M.; Carretero, M.; et al. Efficient CRISPR-Cas9-Mediated Gene Ablation in Human Keratinocytes to Recapitulate Genodermatoses: Modeling of Netherton Syndrome. Mol. Ther. Methods Clin. Dev. 2020, 18, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Chondrocyte sheet in vivo cartilage regeneration technique using miR-193b-3p to target MMP16. Aging 2019, 11, 7070–7082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Whalley, R.D.; Ferreira, A.M.; Dalgano, K. High throughput physiological micro-models for in vitro pre-clinical drug testing: A review of engineering systems approaches. Prog. Biomed. Eng. 2020, 2, 22001. [Google Scholar] [CrossRef]
- Asadi, M.; Lotfi, H.; Salehi, R.; Mehdipour, A.; Zarghami, N.; Akbarzadeh, A.; Alizadeh, E. Hepatic cell-sheet fabrication of differentiated mesenchymal stem cells using decellularized extracellular matrix and thermoresponsive polymer. Biomed. Pharmacother. 2021, 134, 111096. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef]
| Method | Labelling Method | Detection Depth of the Signal (In Vivo) | Invasive Method | Detection of Living Cells | Safe Methodology For Patients | Animal Size | Data Acquisition Time | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Direct Cell Visualization | Ultrasound | Incubation | Whole body | No | Can’t be determinated | Yes | Any Animal | Minutes/Hours | [125,126] |
| MR Fluorescent (X-ray Fluoresence) | Incubation | Whole body | No | Can’t be determinated | Yes | Any Animal | Minutes/Hours | [122,132,135] | |
| Direct Cell Labelling | Fluorescence (e.g., quantum dots, fluorophores) | Incubation | 2–3 mm | No | Can’t be determinated | Potentially Toxic | Small Animals | Secondes/Minutes | [133,139] |
| MRI (e.g., SPIO) | Incubation | Whole body | No | Can’t be determinated | Possible Iron Toxicity Harm | Any Animal | Minutes/Hours | [110] | |
| SPECT (e.g., 111InOx) | Incubation | Whole body | No | Can’t be determinated | Potential Radioactive Harm | Any Animal | Minutes | [138] | |
| PET (e.g., 18F-FDG, 64Cu-PTSM) | Incubation | Whole body | No | Can’t be determinated | Potential Radioactive Harm | Any Animal | Minutes | [124,129] | |
| Genetic Modification of the Cells for Cell Detection | Fluorescence (RFP, GFP) | Stable Transfection | Less than 1 cm depth | No | Can’t be determinated | Potential Cell Toxicity | Small animals | Seconds/Minutes | [131,134] |
| Bioluminescence (Fluc, Rluc) | Stable Transfection + Probe injection | Less than 3 cm depth | No | Cells are alive | Yes | Small animals | Seconds/Minutes | [123,135] | |
| SPECT (NIS, somatostatin) | Stable Transfection + Probe injection | Whole body | No | Cells are alive | Potential Radioactive Harm | Any Animal | Minutes | [127,137] | |
| PET (HSV1-tk, D2R) | Stable Transfection + Probe injection | Whole Body | No | Cells are alive | Potential Radioactive Harm and Potential Immunoreactivity with TK | Any Animal | Minutes | [140,141] | |
| MRI (Transferrin, Ferritin, Tyrosine) | Stable Transfection + Probe injection | Whole body | No | Cells are alive | Possible Iron Toxicity Harm | Any Animal | Minutes/hours | [128,130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, J.; Niihara, Y.; Oliva, J. Development of Multilayer Mesenchymal Stem Cell Cell Sheets. Int. J. Transl. Med. 2021, 1, 4-24. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010002
Ochiai J, Niihara Y, Oliva J. Development of Multilayer Mesenchymal Stem Cell Cell Sheets. International Journal of Translational Medicine. 2021; 1(1):4-24. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010002
Chicago/Turabian StyleOchiai, Jun, Yutaka Niihara, and Joan Oliva. 2021. "Development of Multilayer Mesenchymal Stem Cell Cell Sheets" International Journal of Translational Medicine 1, no. 1: 4-24. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010002





