HGF and VEGF-A and Their Receptors Show Expression and Angiogenic Effects on Human Choroidal Endothelial Cells: Implications for Treatments of Neovascular Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Immunohistochemistry
2.3. Immunocytochemistry
2.4. Western Blotting
2.5. Flow Cytometry
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Proliferation Assay
2.8. Migration (Wound Closure) Assay
2.9. In Vitro Tubule Formation
2.10. Statistical Analysis
3. Results
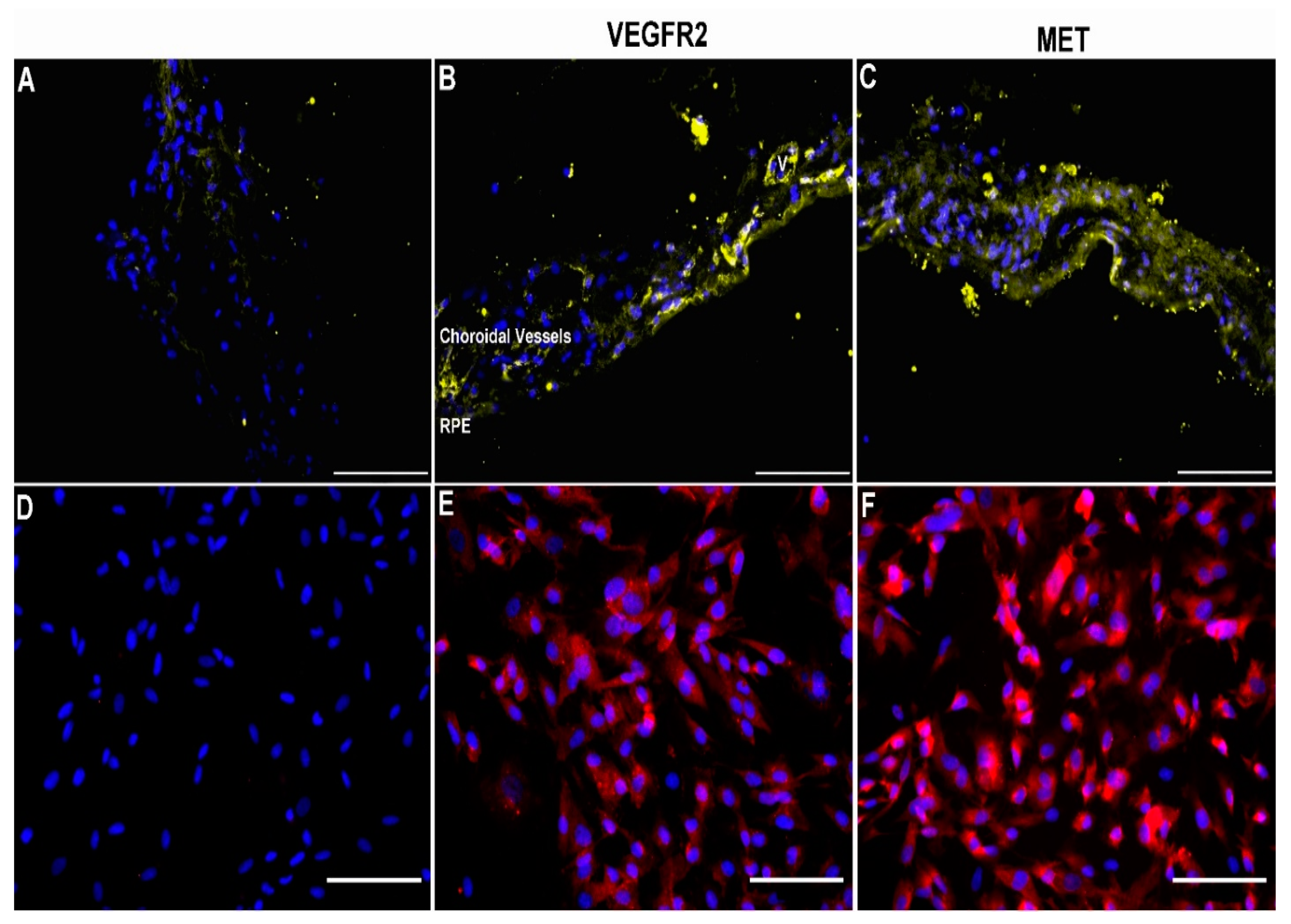
3.1. Expression of VEGFR2 and MET in Human Choroid and Choroidal Endothelial Cells
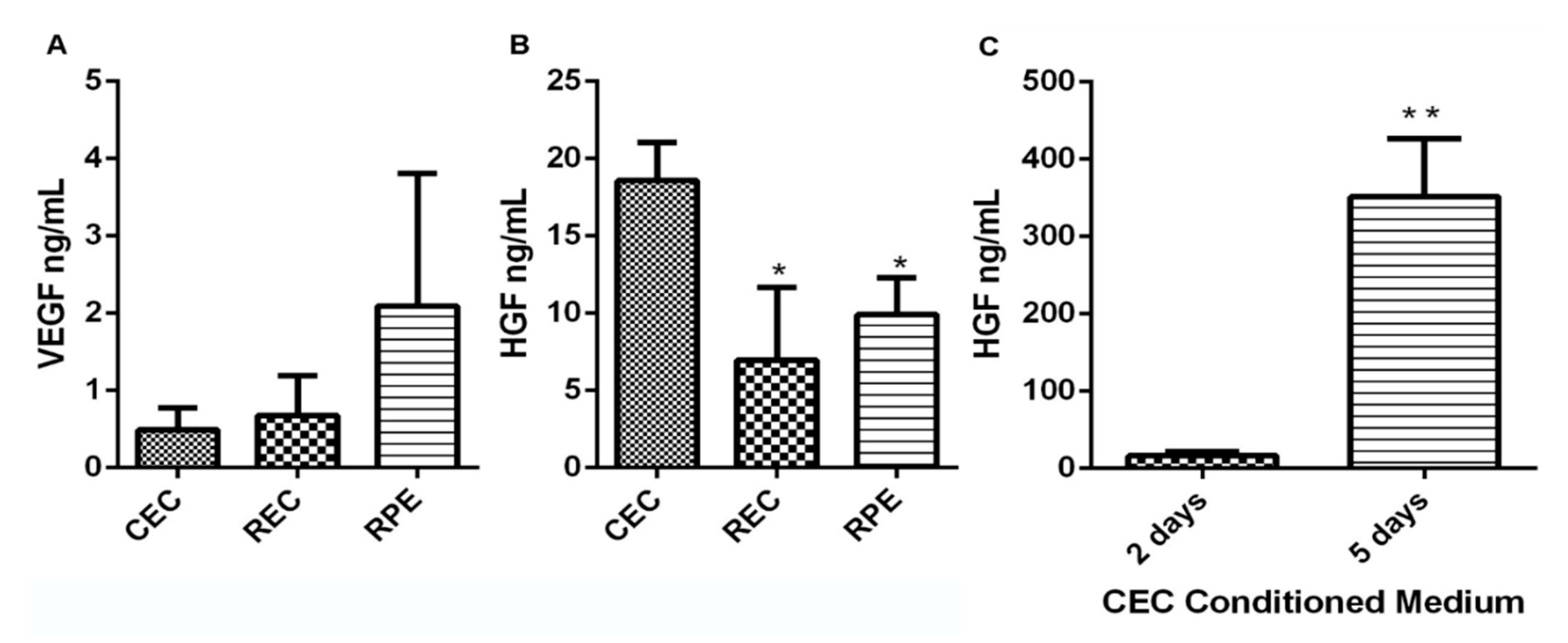
3.2. CEC Secrete High Levels of VEGF and HGF
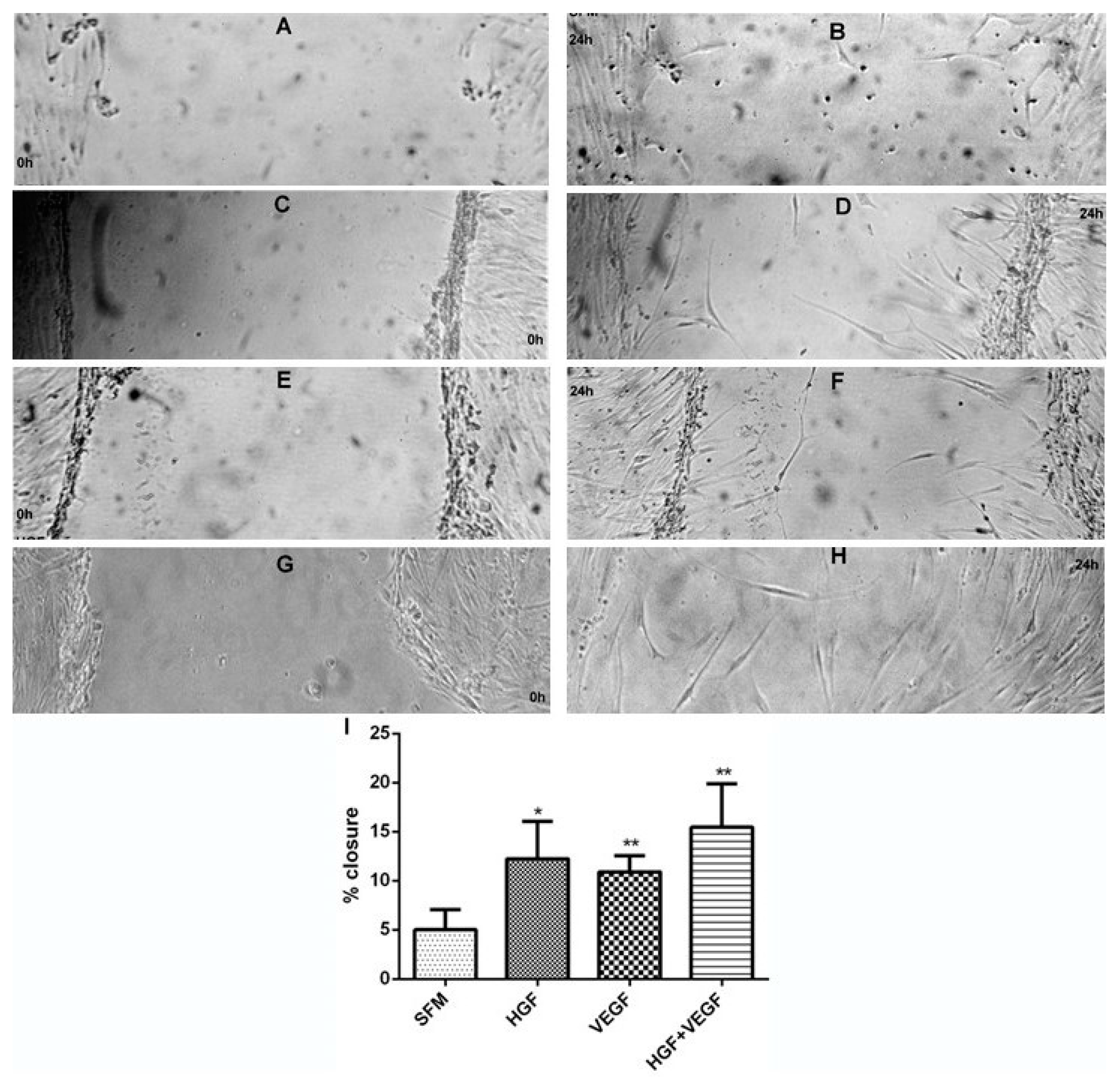
3.3. HGF Stimulation of CEC Proliferation and Migration
3.4. Effect of HGF on CEC Tubule Formation on Matrigel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2019, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Frank, R.N.; Amin, R.H.; Eliott, D.; Puklin, J.E.; Abrams, G.W. Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor Are Present in Epiretinal and Choroidal Neovascular Membranes. Am. J. Ophthalmol. 1996, 122, 393–403. [Google Scholar] [CrossRef]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Cabral, T.; Lima, L.H.; Mello, L.G.M.; Polido, J.; Correa, É.; Oshima, A.; Duong, J.; Serracarbassa, P.; Regatieri, C.V.; Mahajan, V.B.; et al. Bevacizumab Injection in Patients with Neo-vascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol. Retin. 2018, 2, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhu, M.; Xie, L.; Sun, X.; Xu, J.; Guo, Y.; Liu, D.; Shi, Y.; Xu, X.; Song, E. Cabozantinib, a Multityrosine Kinase Inhibitor of MET and VEGF Receptors Which Suppresses Mouse Laser-Induced Choroidal Neovascularization. J. Ophthalmol. 2020, 2020, 5905269. [Google Scholar] [CrossRef]
- Isumi, Y.; Hayashi, S.; Inoue, T.; Yoshigae, Y.; Sato, T.; Hasegawa, J.; Agatsuma, T. DS-7080a, a Selective Anti-ROBO4 Antibody, Shows Anti-Angiogenic Efficacy with Distinctly Different Profiles from Anti-VEGF Agents. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef]
- Bussolino, F.; Di Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, J.S.; Bottaro, D.P.; Aaronson, S. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim. Biophys. Acta 1993, 1155, 357–371. [Google Scholar] [CrossRef]
- Wilson, S.; Walker, J.W.; Chwang, E.L.; He, Y.G. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2544–2561. [Google Scholar]
- Nakamura, Y.; Morishita, R.; Higaki, J.; Kida, I.; Aoki, M.; Moriguchi, A.; Yamada, K.; Hayashi, S.I.; Yo, Y.; Matsumoto, K.; et al. Expression of local hepatocyte growth factor system in vascular tissues. Biochem. Biophys. Res. Commun. 1995, 215, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Emerging Multipotent Aspects of Hepatocyte Growth Factor. J. Biochem. 1996, 119, 591–600. [Google Scholar] [CrossRef]
- Wordinger, R.J.; Clark, A.F.; Agarwal, R.; Lambert, W.; McNatt, L.; Wilson, S.E.; Qu, Z.; Fung, B.K. Cultured human trabecular meshwork cells express functional growth factor receptors. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1575–1589. [Google Scholar]
- Cai, W.; Rook, S.L.; Jiang, Z.Y.; Takahara, N.; Aiello, L.P. Mechanisms of hepatocyte growth factor–induced retinal endothelial cell migration and growth. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1885–1893. [Google Scholar]
- Nishimura, M.; Ikeda, T.; Ushiyama, M.; Nanbu, A.; Kinoshita, S.; Yoshimura, M. Increased Vitreous Concentrations of Human Hepatocyte Growth Factor in Proliferative Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 1999, 84, 659–662. [Google Scholar] [CrossRef]
- Nishimura, M.; Nakano, K.; Ushiyama, M.; Nanbu, A.; Ohtsuka, K.; Takahashi, H.; Yoshimura, M. Increased serum concentrations of human hepatocyte growth factor in proliferative diabetic retinopathy. J. Clin. Endocrinol. Metab. 1998, 83, 195–198. [Google Scholar] [CrossRef]
- Katsura, Y.; Okano, T.; Noritake, M.; Kosano, H.; Nishigori, H.; Kado, S.; Matsuoka, T. Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care 1998, 21, 1759–1763. [Google Scholar] [CrossRef]
- He, P.; He, S.; Garner, J.; Ryan, S.; Hinton, D. Retinal Pigment Epithelial Cells Secrete and Respond to Hepatocyte Growth Factor. Biochem. Biophys. Res. Commun. 1998, 249, 253–257. [Google Scholar] [CrossRef]
- Briggs, M.C.; Grierson, I.; Hiscott, P.; Hunt, J. Active scatter factor (HGF/SF) in proliferative vitreoretinal disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3085–3094. [Google Scholar]
- Liou, G.; Matragoon, S.; Samuel, S.; Behzadian, M.A.; Tsai, N.-T.; Gu, X.; Roon, P.; Hunt, D.M.; Hunt, R.C.; Caldwell, R.B.; et al. MAP kinase and beta-catenin signaling in HGF induced RPE migration. Mol. Vis. 2002, 8, 483–493. [Google Scholar]
- Browning, A.C.; Halligan, E.P.; Stewart, E.A.; Swan, D.; Dove, R.; Samaranayake, G.J.; Amoaku, W.M. Comparative gene expression profiling of human umbilical vein endothelial cells and ocular vascular endothelial cells. Br. J. Ophthalmol. 2011, 96, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, S.; Wu, Q.; Hu, J.; Li, T. Decorin inhibits angiogenic potential of choroid-retinal endothelial cells by downregulating hypoxia-induced Met, Rac1, HIF-1α and VEGF expression in cocultured retinal pigment epithelial cells. Exp. Eye Res. 2013, 116, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial cell heterogeneity. Crit. Care Med. 2003, 31, S221–S230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, A.C.; Dua, H.S.; Amoaku, W.M. The effects of growth factors on the proliferation and in vitro angiogenesis of human macular inner choroidal endothelial cells. Br. J. Ophthalmol. 2008, 92, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Samaranayake, G.J.; Browning, A.C.; Hopkinson, A.; Amoaku, W.M. Comparison of choroidal and retinal endothelial cells: Characteristics and response to VEGF isoforms and anti-VEGF treatments. Exp. Eye Res. 2011, 93, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Browning, A.C.; Gray, T.; Amoaku, W.M. Isolation, culture, and characterisation of human macular inner choroidal microvascular endothelial cells. Br. J. Ophthalmol. 2005, 89, 1343–1347. [Google Scholar] [CrossRef] [Green Version]
- Jo, N.; Mailhos, C.; Ju, M.; Cheung, E.; Bradley, J.; Nishijima, K.; Robinson, G.S.; Adamis, A.P.; Shima, D.T. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am. J. Pathol. 2006, 168, 2036–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Criswell, M.H.; Fong, S.-L.; Temm, C.J.; Rajashekhar, G.; Cornell, T.L.; Clauss, M.A. Differences in the temporal expression of regulatory growth factors during choroidal neovascular development. Exp. Eye Res. 2009, 88, 79–91. [Google Scholar] [CrossRef]
- Abounader, R.; Laterra, J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro. Oncol. 2005, 7, 436–451. [Google Scholar] [CrossRef]
- Kuba, K.; Matsumoto, K.; Date, K.; Shimura, H.; Tanaka, M.; Nakamura, T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000, 60, 6737–6743. [Google Scholar] [PubMed]
- Li, Y.; Lal, B.; Kwon, S.; Fan, X.; Saldanha, U.; Reznik, T.E.; Kuchner, E.B.; Eberhart, C.; Laterra, J.; Abounader, R. The scatter factor/hepatocyte growth factor: C-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005, 65, 9355–9362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.W.; Wang, L.M.; Jove, R.; Woude, G.F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 2002, 21, 217–226. [Google Scholar] [CrossRef]
- Du, T.; Zou, X.; Cheng, J.; Wu, S.; Zhong, L.; Ju, G.; Zhu, J.; Liu, G.; Zhu, Y.; Xia, S. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res. Ther. 2013, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol. 2012, 90, e381–e388. [Google Scholar] [CrossRef] [PubMed]
- Lashkari, K.; Rahimi, N.; Kazlauskas, A. Hepatocyte growth factor receptor in human RPE cells: Implications in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1999, 40, 149–156. [Google Scholar]
- Grierson, I.; Heathcote, L.; Hiscott, P.; Hogg, P.; Briggs, M.; Hagan, S. Hepatocyte growth factor/Scatter factor in the eye. Prog. Retin. Eye Res. 2000, 19, 779–802. [Google Scholar] [CrossRef]
- Yoshida, S.; Ishikawa, K.; Matsumoto, T.; Yoshida, A.; Ishibashi, T.; Kono, T. Reduced concentrations of angiogenesis-related factors in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 799–804. [Google Scholar] [CrossRef]
- Zheng, X.; Peng, Q.; Wang, L.; Zhang, X.; Huang, L.; Wang, J.; Qin, Z. Serine/arginine-rich splicing factors: The bridge linking alternative splicing and cancer. Int. J. Biol. Sci. 2020, 16, 2442. [Google Scholar] [CrossRef]
- Cao, S.; Walker, G.B.; Wang, X.; Cui, J.Z.; Matsubara, J.A. Altered cytokine profiles of human retinal pigment epithelium: Oxidant injury and replicative senescence. Mol. Vis. 2013, 19, 718–728. [Google Scholar]
- Keyt, B.A.; Berleau, L.T.; Nguyen, H.V.; Chen, H.; Heinsohn, H.; Vandlen, R.; Ferrara, N. The Carboxyl-terminal Domain (111–165) of Vascular Endothelial Growth Factor Is Critical for Its Mitogenic Potency. J. Biol. Chem. 1996, 271, 7788–7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Amano, S.; Ogura, Y.; Hida, T.; Oguchi, Y.; Ambati, J.; Miller, J.W.; et al. VEGF164-mediated Inflammation Is Required for Pathological, but Not Physiological, Ischemia-induced Retinal Neovascularization. J. Exp. Med. 2003, 198, 483–489. [Google Scholar] [CrossRef]
- Bhisitkul, R.B. Vascular endothelial growth factor biology: Clinical implications for ocular treatments. Br. J. Ophthalmol. 2006, 90, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Gherardi, E.; Sellers, L.A.; Wood, J.M.; Sasisekharan, R.; Fan, T.-P.D. Hepatocyte growth factor/scatter factor can induce angiogenesis independently of vascular endothelial growth factor. Arter. Thromb. Vasc. Biol. 2003, 23, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-W.; Su, Y.; Volpert, O.V.; Woude, G.F.V. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc. Natl. Acad. Sci. USA 2003, 100, 12718–12723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulpice, E.; Ding, S.; Muscatelli-Groux, B.; Bergé, M.; Han, Z.C.; Plouet, J.; Tobelem, G.; Merkulova-Rainon, T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol. Cell 2009, 101, 525–539. [Google Scholar] [CrossRef]
- D’Amore, P.A. Vascular Endothelial Cell Growth Factor-A: Not Just for Endothelial Cells Anymore. Am. J. Pathol. 2007, 171, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular Endothelial Growth Factor-A Is a Survival Factor for Retinal Neurons and a Critical Neuroprotectant during the Adaptive Response to Ischemic Injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Blouw, B.; Song, H.; Tihan, T.; Bosze, J.; Ferrara, N.; Gerber, H.-P.; Johnson, R.S.; Bergers, G. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 2003, 4, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Ebos, J.M.; Bocci, G.; Man, S.; Thorpe, P.E.; Hicklin, D.J.; Zhou, D.; Jia, X.; Kerbel, R.S. A Naturally Occurring Soluble Form of Vascular Endothelial Growth Factor Receptor 2 Detected in Mouse and Human Plasma. Mol. Cancer Res. 2004, 2, 315–326. [Google Scholar] [PubMed]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A.; et al. VEGF Inhibits Tumor Cell Invasion and Mesenchymal Transition through a MET/VEGFR2 Complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, C.; Davies, G.; Nakamura, T.; Matsumoto, K.; Mason, M.; Jiang, W. The HGF/SF-Induced Phosphorylation of Paxillin, Matrix Adhesion, and Invasion of Prostate Cancer Cells Were Suppressed by NK4, an HGF/SF Variant. Biochem. Biophys. Res. Commun. 2001, 285, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, H.; Zheng, Y.; Gu, Q.; Ma, J.; Xu, X. A novel antiangiogenic peptide derived from hepatocyte growth factor inhibits neovascularization in vitro and in vivo. Mol. Vis. 2010, 16, 1982–1995. [Google Scholar] [PubMed]
- Wen, P.Y.; Schiff, D.; Cloughesy, T.F.; Raizer, J.J.; Laterra, J.; Smitt, M.; Wolf, M.; Oliner, K.S.; Anderson, A.; Zhu, M.; et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011, 13, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Jun, H.T.; Sun, J.; Rex, K.; Radinsky, R.; Kendall, R.; Coxon, A.; Burgess, T.L. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin. Cancer Res. 2007, 13, 6735–6742. [Google Scholar] [CrossRef] [Green Version]
- Neill, T.; Painter, H.; Buraschi, S.; Owens, R.T.; Lisanti, M.P.; Schaefer, L.; Iozzo, R.V. Decorin antagonizes the angiogenic network: Concurrent inhibition of Met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. J. Biol. Chem. 2012, 287, 5492–5506. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, E.A.; Allen, C.L.; Samaranayake, G.J.; Stubington, T.; Akhtar, R.; Branch, M.J.; Amoaku, W.M. HGF and VEGF-A and Their Receptors Show Expression and Angiogenic Effects on Human Choroidal Endothelial Cells: Implications for Treatments of Neovascular Age-Related Macular Degeneration. Int. J. Transl. Med. 2021, 1, 69-82. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010006
Stewart EA, Allen CL, Samaranayake GJ, Stubington T, Akhtar R, Branch MJ, Amoaku WM. HGF and VEGF-A and Their Receptors Show Expression and Angiogenic Effects on Human Choroidal Endothelial Cells: Implications for Treatments of Neovascular Age-Related Macular Degeneration. International Journal of Translational Medicine. 2021; 1(1):69-82. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010006
Chicago/Turabian StyleStewart, Elizabeth A., Claire L. Allen, Govindi J. Samaranayake, Thomas Stubington, Rukhsar Akhtar, Matthew J. Branch, and Winfried M. Amoaku. 2021. "HGF and VEGF-A and Their Receptors Show Expression and Angiogenic Effects on Human Choroidal Endothelial Cells: Implications for Treatments of Neovascular Age-Related Macular Degeneration" International Journal of Translational Medicine 1, no. 1: 69-82. https://0-doi-org.brum.beds.ac.uk/10.3390/ijtm1010006




