Modeling and Thermodynamic Analysis of the Water Sorption Isotherms of Cottonseed Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorption Isotherms
2.3. Mathematical Modelling
3. Results and Discussion
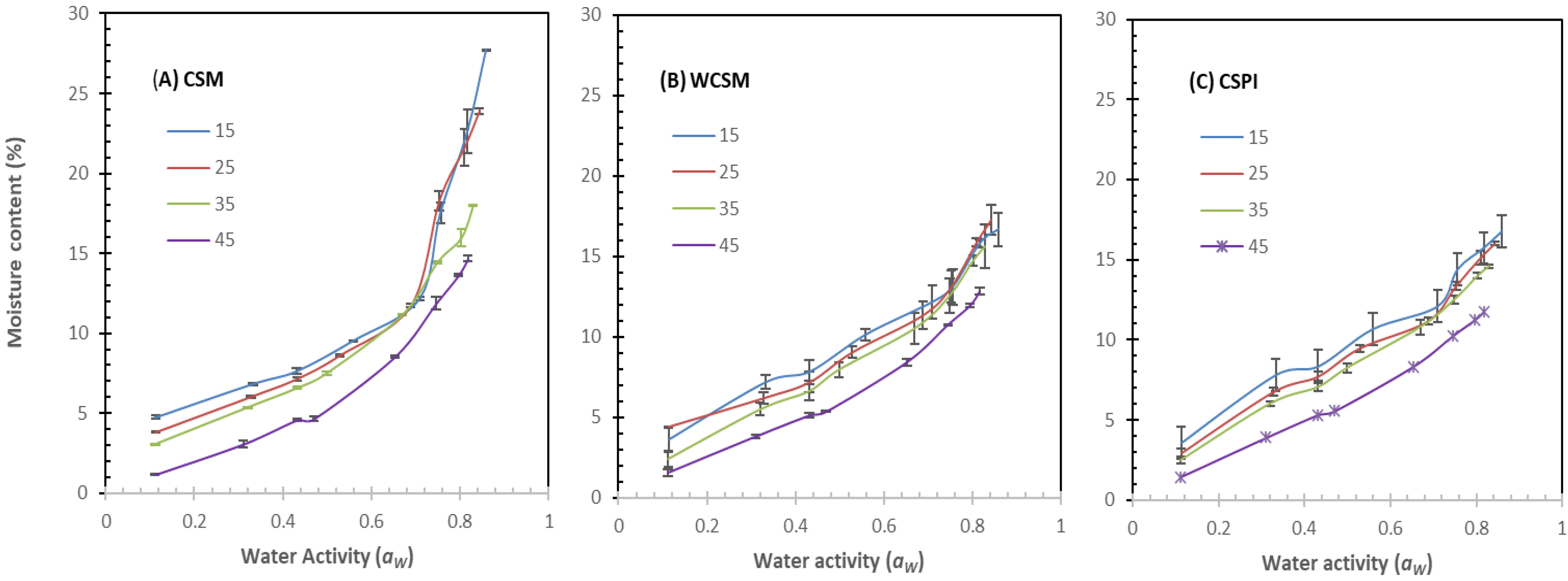
3.1. Experimental Moisture Sorption Isotherms
3.2. Modeling Adsorption Isotherms
3.3. Monolayer Moisture Content and Specific Surface Area of Sorption
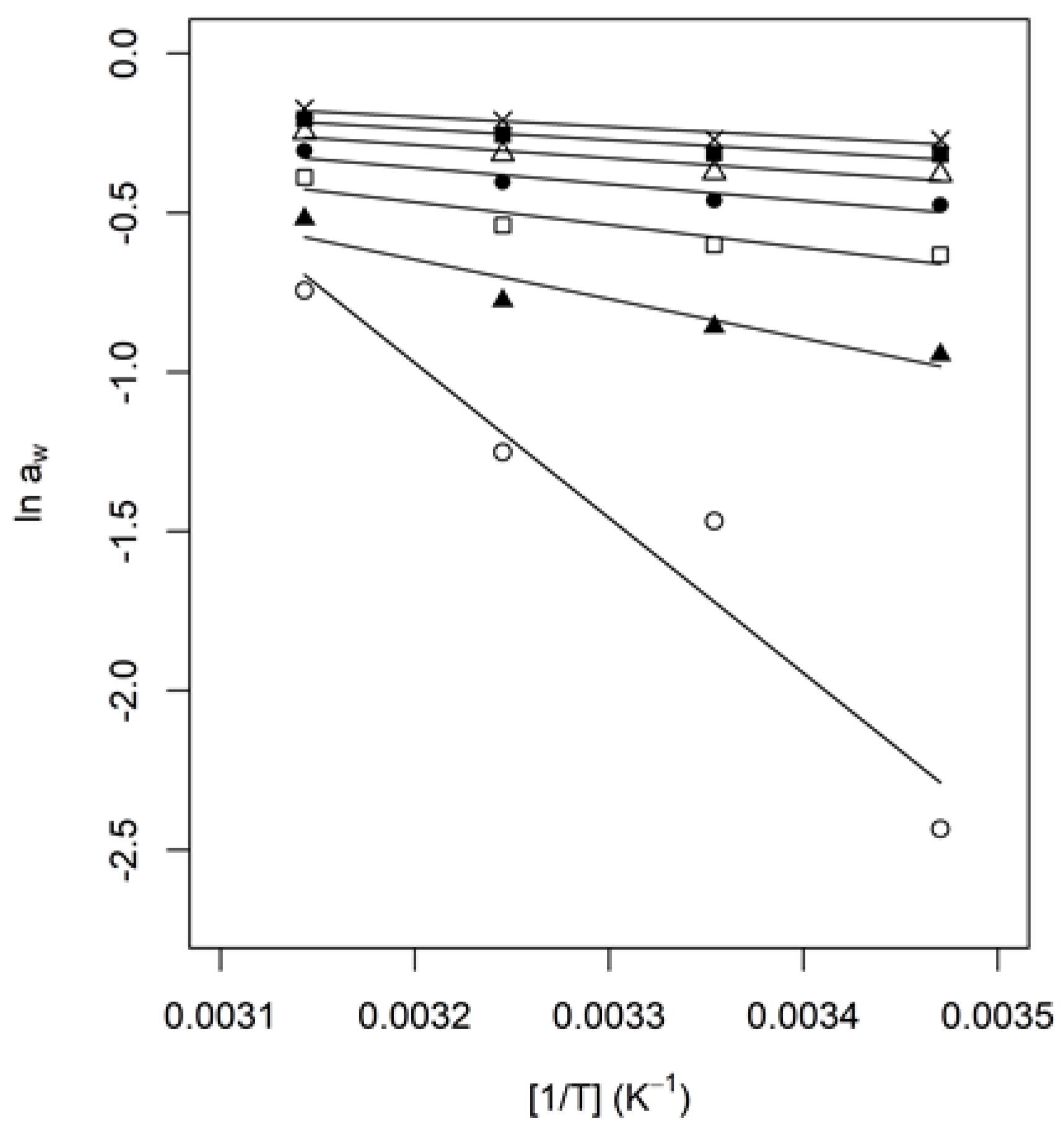
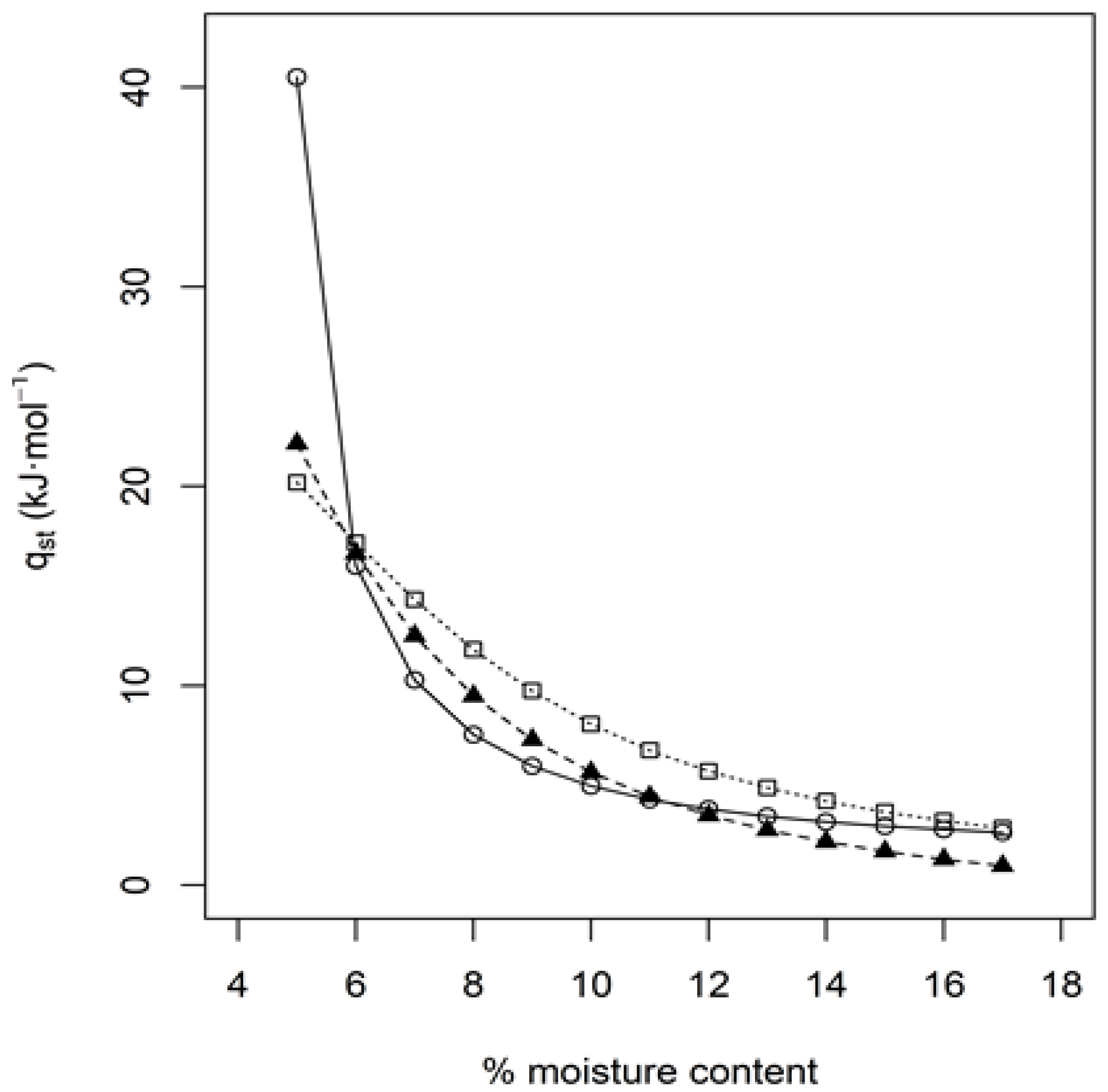
3.4. Thermodynamic Properties
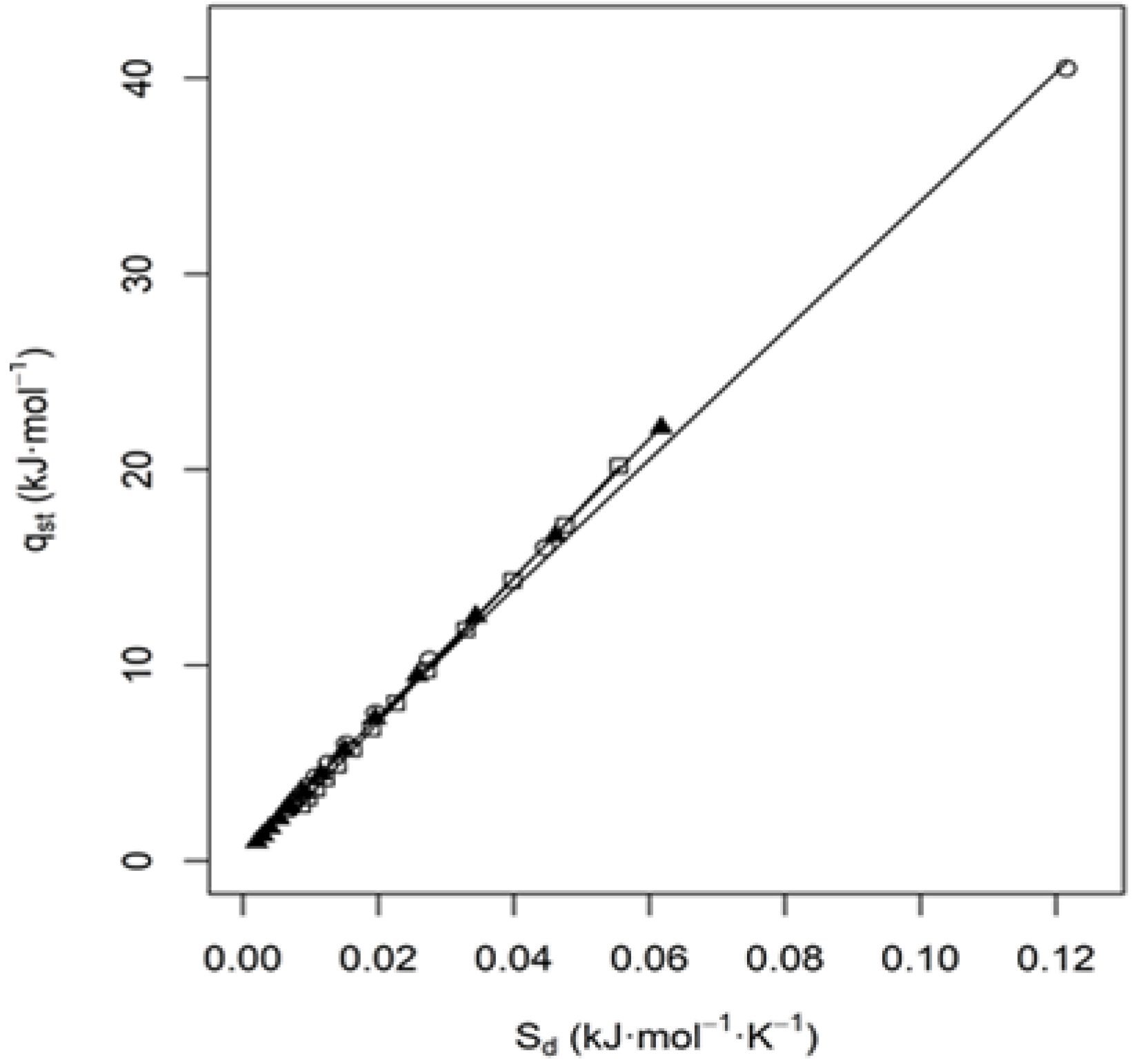
3.5. Entropy—Enthalpy Compensation Theory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Z.; Zhang, H.; Fang, D.D.; Zeng, L.; Jenkins, J.N.; McCarty, J.C. Effects of inter-species chromosome substitution on cottonseed mineral and protein nutrition profiles. Agron. J. 2020, 112, 3963–3974. [Google Scholar] [CrossRef]
- Tewolde, H.; Shankle, M.W.; Way, T.R.; Pote, D.H.; Sistani, K.R.; He, Z. Poultry litter band placement affects accessibility and conservation of nutrients and cotton yield. Agron. J. 2018, 110, 675–684. [Google Scholar] [CrossRef]
- Amin, A.; Nasim, W.; Mubeen, M.; Ahmad, A.; Nadeem, M.; Urich, P.; Fahad, S.; Ahmad, S.; Wajid, A.; Tabassum, F. Simulated csm-cropgro-cotton yield under projected future climate by simclim for southern punjab, pakistan. Agric. Syst. 2018, 167, 213–222. [Google Scholar] [CrossRef]
- Basal, H.; Karademir, E.; Goren, H.K.; Sezener, V.; Dogan, M.N.; Gencsoylu, I.; Erdogan, O. Cotton production in turkey and europe. In Cotton Production; Jabran, K., Chauhan, B.S., Eds.; John Wiley & Son, Inc.: New York, NY, USA, 2019; pp. 297–321. [Google Scholar]
- Zhang, G.; Zhang, W.; Pu, X.; Zhang, P.; Ou, Y. Influence of stalk residue retention and fertilization on the rhizospheric effect with drip-irrigated cotton. Agron. J. 2019, 111, 1028–1038. [Google Scholar] [CrossRef]
- He, Z.; Mattison, C.P.; Zhang, D.; Grimm, C.C. Vicilin and legumin storage proteins are abundant in water and alkali soluble protein fractions of glandless cottonseed. Sci. Rep. 2021, 11, 9209. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- He, Z.; Uchimiya, S.M.; Guo, M. Production and characterization of biochar from agricultural by-products: Overview and use of cotton biomass residues. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 63–86. [Google Scholar]
- Al Afif, R.; Anayah, S.S.; Pfeifer, C. Batch pyrolysis of cotton stalks for evaluation of biochar energy potential. Renew. Energy 2020, 147, 2250–2258. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.N.; He, Z.; Ford, C.; Wyckoff, W.; Wu, Q. A review of cottonseed protein chemistry and non-food applications. Sustain. Chem. 2020, 1, 17. [Google Scholar] [CrossRef]
- He, Z.; Olk, D.C.; Tewolde, H.; Zhang, H.; Shankle, M. Carbohydrate and amino acid profiles of cotton plant biomass products. Agriculture 2020, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Gutiérrez, E.; Buenrostro-Figueroa, J.; López-Martínez, L.; Sepúlveda, D.; Baeza-Jiménez, R. Biotechnological potential of cottonseed, a by-product of cotton production. In Valorisation of Agro-Industrial Residues–Volume II: Non-Biological Approaches; Springer: Berlin, Germany, 2020; pp. 63–82. [Google Scholar]
- Tunc, S.; Duman, O. Thermodynamic properties and moisture adsorption isotherms of cottonseed protein isolate and different forms of cottonseed samples. J. Food Eng. 2007, 81, 133–143. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N. Preparation and utilization of water washed cottonseed meal as wood adhesives. In Bio-Based Wood Adhesives: Preparation, Characterization and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 156–178. [Google Scholar]
- Swiatkiewicz, S.; Arczewska-Wlosek, A.; Jozefia, D. The use of cottonseed meal as a protein source for poultry: An updated review. World Poult. Sci. J. 2016, 72, 473–484. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Castaño, J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Gallardo-Rivera, R.; Pérez-Alonso, C.; Guadarrama-Lezama, A. Thermodynamic sorption analysis and glass transition temperature of faba bean (Vicia faba L.) protein. J. Food Sci. Technol. 2018, 55, 935–943. [Google Scholar] [CrossRef]
- Jian, F.; Jayas, D.S.; Fields, P.G.; White, N.D. Water sorption and cooking time of red kidney beans (Phaseolus vulgaris L.): Part ii–mathematical models of water sorption. Int. J. Food Sci. Technol. 2017, 52, 2412–2421. [Google Scholar] [CrossRef]
- Soleimanifard, S.; Hamdami, N. Modelling of the sorption isotherms and determination of the isosteric heat of split pistachios, pistachio kernels and shells. Czech J. Food Sci. 2018, 36, 268–275. [Google Scholar] [CrossRef]
- Taitano, L.; Singh, R.; Lee, J.; Kong, F. Thermodynamic analysis of moisture adsorption isotherms of raw and blanched almonds. J. Food Process Eng. 2012, 35, 840–850. [Google Scholar] [CrossRef]
- Arslan-Tontul, S. Moisture sorption isotherm, isosteric heat and adsorption surface area of whole chia seeds. LWT 2020, 119, 108859. [Google Scholar] [CrossRef]
- Moussaoui, H.; Kouhila, M.; Lamsyehe, H.; Idlimam, A.; Lamharrar, A. Moisture sorption measurements and thermophysical characterization of the taraxacum officinale leaves and root. Heat Mass Transfer 2020, 56, 2065–2077. [Google Scholar] [CrossRef]
- Abdenouri, N.; Idlimam, A.; Kouhila, M. Sorption isotherms and thermodynamic properties of powdered milk. Chem. Eng. Comm. 2010, 197, 1109–1125. [Google Scholar] [CrossRef]
- Hssaini, L.; Ouaabou, R.; Charafi, J.; Idlimam, A.; Lamharrar, A.; Razouk, R.; Hanine, H. Hygroscopic proprieties of fig (Ficus carica L.): Mathematical modelling of moisture sorption isotherms and isosteric heat kinetics. South Afr. J. Bot. 2020, in press. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Galvez, A.; Sanders, M.; Lopez, J.; Lemus-Mondaca, R.; Martinez, E.; Di Scala, K. Modelling the water sorption isotherms of quinoa seeds (Chenopodium quinoa willd.) and determination of sorption heats. Food Bioprocess Technol. 2012, 5, 1686–1693. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Kouhila, M.; Kechaou, N. Sorption isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): Experimental and mathematical investigations. Food Bioprod. Process. 2008, 86, 165–167. [Google Scholar]
- Chen, N.; Huang, J.; Li, K. Investigation of a formaldehyde-free cottonseed flour-based adhesive for interior plywood. BioResources 2020, 15, 5546–5557. [Google Scholar]
- Li, J.; Pradyawong, S.; He, Z.; Sun, X.S.; Wang, D.; Cheng, H.N.; Zhong, J. Assessment and application of phosphorus/calcium-cottonseed protein adhesive for plywood production. J. Clean. Prod. 2019, 229, 454–462. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- He, Z.; Chiozza, F. Adhesive strength of pilot-scale-produced water-washed cottonseed meal in comparison with a synthetic glue for non-structural interior application. J. Mater. Sci. Res. 2017, 6, 20–26. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Klasson, K.T.; Olanya, M.O.; Uknalis, J. Application of tung oil to improve adhesion strength and water resistance of cottonseed meal and protein adhesives on maple veneer. Ind. Crop. Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Li, J.; Pradyawong, S.; Sun, X.S.; Wang, D.; He, Z.; Zhong, J.; Cheng, H.N. Improving adhesion performance of cottonseed protein by the synergy of phosphoric acid and water soluble calcium salts. Int. J. Adhes. Adhes. 2021, 108, 102867. [Google Scholar] [CrossRef]
- He, Z.; Klasson, K.T.; Wang, D.; Li, N.; Zhang, H.; Zhang, D.; Wedegaertner, T.C. Pilot-scale production of washed cottonseed meal and co-products. Mod. Appl. Sci. 2016, 10, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Aviara, N.A. Moisture sorption isotherms and isotherm model performance evaluation for food and agricultural products. In Sorption in 2020s; IntechOpen: London, UK, 2020; p. 145. [Google Scholar]
- Bakhtavar, M.A.; Afzal, I.; Basra, S.M.A. Moisture adsorption isotherms and quality of seeds stored in conventional packaging materials and hermetic super bag. PLoS ONE 2019, 14, e0207569. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Shivhare, U.; Mujumdar, A. Models for sorption isotherms for foods: A review. Dry. Technol. 2006, 24, 917–930. [Google Scholar] [CrossRef]
- Kaya, S.; Kahyaoglu, T. Thermodynamic properties and sorption equilibrium of pestil. J. Food Eng. 2005, 71, 200–207. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Mathematical modelling of water sorption isotherms and thermodynamic properties of cucumis melo l. Seeds. LWT 2020, 131, 109727. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, R.; Singh, A.; Patel, A.; Patil, G. Sorption isotherms and thermodynamics of water sorption of ready-to-use basundi mix. LWT 2009, 42, 441–445. [Google Scholar] [CrossRef]
- Verbeek, C.J.; Koppel, N.J. Moisture sorption and plasticization of bloodmeal-based thermoplastics. J. Mater. Sci. 2012, 47, 1187–1195. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Water sorption and thermo-mechanical properties of water/sorbitol-plasticized composite biopolymer films: Caseinate–pullulan bilayers and blends. Food Hydrocoll. 2006, 20, 1057–1071. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Olanya, O.M.; Uknalis, J.; Zhang, X.; Koplitz, B.D.; He, J. Surface characterization of cottonseed meal products by sem, sem-eds, xrd and xps analysis. J. Mater. Sci. Res. 2018, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Uchimiya, M.; Cao, H. Intrinsic fluorescence excitation-emission matrix spectral features of cottonseed protein fractions and the effects of denaturants. J. Am. Oil Chem. Soc. 2014, 91, 1489–1497. [Google Scholar] [CrossRef]
- Corrêa, P.; Goneli, A.; Jaren, C.; Ribeiro, D.; Resende, O. Sorption isotherms and isosteric heat of peanut pods, kernels and hulls. Food Sci. Technol. Int. 2007, 13, 231–238. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gołembiewski, R.; Gauden, P.A.; Czepirski, L. Searching the most optimal model of water sorption on foodstuffs in the whole range of relative humidity. Food Res. Int. 2009, 42, 1203–1214. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Samapundo, S.; Devlieghere, F.; De Saeger, S.; De Meulenaer, B. Moisture sorption isotherms and thermodynamic properties of whole black peppercorns (Piper nigrum L.). LWT 2015, 64, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Ocieczek, A.; Skotnicka, M.; Baranowska, K. Sorptive properties of modified maize starch as indicators of their quality. Int. Agrophys. 2017, 31, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Timmermann, E.O.; Chirife, J.; Iglesias, H.A. Water sorption isotherms of foods and foodstuffs: Bet and gab parameters. J. Food Eng. 2001, 48, 19–31. [Google Scholar] [CrossRef]
- Ariahu, C.C.; Kaze, S.A.; Achem, C.D. Moisture sorption characteristics of tropical fresh water crayfish (Procam barus clarkia). J. Food Eng. 2005, 75, 355–363. [Google Scholar] [CrossRef]
- Bajpai, S.; Pradeep, T. Studies on equilibrium moisture absorption of kappa carrageenan. Int. Food Res. J. 2013, 20, 2183. [Google Scholar]
- He, Z.; Cheng, H.N.; Chapital, D.C.; Dowd, M.K. Sequential fractionation of cottonseed meal to improve its wood adhesive properties. J. Am. Oil Chem. Soc. 2014, 91, 151–158. [Google Scholar] [CrossRef]
- Cassini, A.S.; Marczak, L.D.F.; Norena, C.P.Z. Water adsorption isotherms of texturized soy protein. J. Food Eng. 2006, 77, 194–199. [Google Scholar] [CrossRef]
- Labuza, T.P. Sorption phenomena in foods. Food Technol. 1968, 22, 263–272. [Google Scholar]
- Labuza, T.; Kaanane, A.; Chen, J. Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J. Food Sci. 1985, 50, 385–392. [Google Scholar] [CrossRef]
- McMinn, W.; Al-Muhtaseb, A.; Magee, T. Enthalpy–entropy compensation in sorption phenomena of starch materials. Food Res. Int. 2005, 38, 505–510. [Google Scholar] [CrossRef]
- Olsson, T.S.; Ladbury, J.E.; Pitt, W.R.; Williams, M.A. Extent of enthalpy–entropy compensation in protein–ligand interactions. Protein Sci. 2011, 20, 1607–1618. [Google Scholar] [CrossRef] [Green Version]
- Bastıoğlu, A.Z.; Koç, M.; Ertekin, F.K. Moisture sorption isotherm of microencapsulated extra virgin olive oil by spray drying. J. Food Measur. Character. 2017, 11, 1295–1305. [Google Scholar] [CrossRef]

| Protein | Oil | Cellulose | Ash | P | Ca | K | Mg | Na | S | |
|---|---|---|---|---|---|---|---|---|---|---|
| % of product weight | ||||||||||
| CSM | 34.1 | 2.5 | 13.6 | 7.2 | 1.5 | 0.3 | 1.8 | 0.7 | 0.2 | 0.5 |
| WCSM | 46.3 | 1 | 17.6 | 5.2 | 1.2 | 0.3 | 1 | 0.7 | 0.1 | 0.5 |
| CSPI | 94.8 | 0.2 | 0.6 | 2.2 | 0.5 | <0.1 | 0.3 | 0.1 | 0.2 | 0.7 |
| Cottonseed Meal. | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G.A.B. | B.E.T. | Halsey | |||||||||||||||||
| Temp (°C) | Mo | C | K | R2 | P | SE | Mm | C | R2 | P | SE | a’’ | r | R2 | P | SE | |||
| 15 | 4.302 | −180.9 | 0.9779 | 0.9312 | 5.419 | 1.173 | 3.961 | −30.46 | 0.9736 | 8.390 | 1.079 | 19.04 | 1.494 | 0.9733 | 8.224 | 1.637 | |||
| 25 | 4.227 | 36.03 | 0.9868 | 0.9422 | 4.380 | 1.233 | 4.008 | 143.3 | 0.9660 | 6.193 | 1.231 | 12.41 | 1.349 | 0.9874 | 5.532 | 1.138 | |||
| 35 | 4.443 | 13.65 | 0.9190 | 0.9863 | 1.590 | 0.3967 | 3.334 | −31.78 | 0.9617 | 13.93 | 1.294 | 11.36 | 1.400 | 0.9953 | 3.712 | 0.5454 | |||
| 45 | 3.866 | 3.114 | 0.9399 | 0.9779 | 2.731 | 0.3870 | 3.037 | 4.989 | 0.9511 | 6.052 | 0.6915 | 3.180 | 0.9773 | 0.9760 | 11.52 | 1.107 | |||
| Washed Cottonseed Meal. | |||||||||||||||||||
| G.A.B. | Henderson | Oswin | |||||||||||||||||
| Temp (°C) | Mo | C | K | R2 | P | SE | k | n | R2 | P | SE | a | n | R2 | P | SE | |||
| 15 | 6.591 | 11.92 | 0.7231 | 0.9589 | 3.010 | 0.4485 | 0.01137 | 1.848 | 0.9939 | 3.262 | 0.5126 | 19.04 | 1.494 | 0.9733 | 8.224 | 1.637 | |||
| 25 | 4.829 | 42.36 | 0.8564 | 0.9812 | 2.112 | 0.3119 | 0.008515 | 1.979 | 0.9427 | 9.508 | 1.266 | 12.41 | 1.349 | 0.9874 | 5.532 | 1.138 | |||
| 35 | 5.899 | 6.404 | 0.7812 | 0.9502 | 2.101 | 0.2642 | 0.03287 | 1.467 | 0.9973 | 2.636 | 0.3800 | 11.36 | 1.400 | 0.9953 | 3.712 | 0.5454 | |||
| 45 | 4.542 | 4.486 | 0.8334 | 0.9719 | 1.403 | 0.1518 | 0.06921 | 1.268 | 0.9978 | 2.689 | 0.2601 | 3.180 | 0.9773 | 0.9760 | 11.52 | 1.107 | |||
| Cottonseed Protein Isolate. | |||||||||||||||||||
| G.A.B. | Bradley | Henderson | |||||||||||||||||
| Temp (°C) | Mo | C | K | R2 | P | SE | K1 | K2 | R2 | P | SE | k | n | R2 | P | SE | |||
| 15 | 7.755 | 9.812 | 0.6590 | 0.9254 | 3.584 | 0.5635 | 0.8151 | 4.832 | 0.9888 | 4.248 | 0.5108 | 0.01066 | 1.849 | 0.9906 | 3.551 | 0.5296 | |||
| 25 | 7.338 | 7.384 | 0.6816 | 0.9107 | 2.927 | 0.4585 | 0.8201 | 3.983 | 0.9938 | 2.706 | 0.3784 | 0.01883 | 1.659 | 0.9940 | 3.002 | 0.4242 | |||
| 35 | 7.451 | 5.595 | 0.6648 | 0.9370 | 1.745 | 0.2017 | 0.8165 | 3.647 | 0.9989 | 1.592 | 0.1547 | 0.02776 | 1.540 | 0.9960 | 2.491 | 0.2517 | |||
| 45 | 6.474 | 3.178 | 0.6834 | 0.9272 | 1.286 | 0.1161 | 0.7975 | 2.857 | 0.9975 | 3.986 | 0.2022 | 0.06995 | 1.281 | 0.9978 | 2.482 | 0.2027 | |||
| Sample | Temp (°C) | S0 (m2g−1) |
|---|---|---|
| Cottonseed meal | 15 | 152.5 |
| 25 | 149.9 | |
| 35 | 157.5 | |
| 45 | 137.0 | |
| Washed cottonseed meal | 15 | 233.7 |
| 25 | 171.2 | |
| 35 | 209.1 | |
| 45 | 161.0 | |
| Cottonseed protein isolate | 15 | 274.9 |
| 25 | 260.1 | |
| 35 | 264.1 | |
| 45 | 229.5 |
| Sample | Tβ (K) | α (kJ∙mol−1) | R2 |
|---|---|---|---|
| Cottonseed Meal | 329.7 | 0.7418 | 0.9991 |
| Washed Cottonseed Meal | 354.2 | 0.3279 | 0.9999 |
| Cottonseed Protein Isolate | 368.0 | −0.2692 | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, D.; Cheng, H.N. Modeling and Thermodynamic Analysis of the Water Sorption Isotherms of Cottonseed Products. Foundations 2021, 1, 32-44. https://0-doi-org.brum.beds.ac.uk/10.3390/foundations1010005
He Z, Zhang D, Cheng HN. Modeling and Thermodynamic Analysis of the Water Sorption Isotherms of Cottonseed Products. Foundations. 2021; 1(1):32-44. https://0-doi-org.brum.beds.ac.uk/10.3390/foundations1010005
Chicago/Turabian StyleHe, Zhongqi, David Zhang, and Huai N. Cheng. 2021. "Modeling and Thermodynamic Analysis of the Water Sorption Isotherms of Cottonseed Products" Foundations 1, no. 1: 32-44. https://0-doi-org.brum.beds.ac.uk/10.3390/foundations1010005







