1. Introduction
Medicinal Plants are the basis of the discovery of new bioactive molecules leading and inspiring the pharmaceutical industry toward the elaboration of more powerful and accurate medicines [
1]. Herbal preparation gained major popularity nowadays because of the fast information spread along with scientific studies confirming their traditional use and being a safe alternative to conventional drugs [
2]. Along with this new wave, countries worked to preserve and organize the information about their medicinal plants through the publishment of several monographs and the elaboration of multiple pharmacopeias [
3]. To assure their correct usage many countries have precisely defined some legal regulation that has to be made in order to overcome three key points: quality, efficacy and safety of any commercialized herbal preparation in the market making the safeguard of the public health their ultimate objective [
4]. Several important point regulations of herbal medicine in a legal environment have also been implemented in a number of nations and areas.
The Committee on Herbal Medicinal Products (HMPC) developed monographs within the EU to cover all elements of the quality, safety, and effectiveness, as well as the regulation of herbal medicine, under Directive 2004/24/EC.
Following the implementation of the traditional herbal medicinal products directive, the herbal medicine products were clearly defined into three categories, the first category includes new herbal medicinal products (HMPs) where their marketing follows the same rules for other medicinal products, the second category comprises well-established use HMPs who require an appropriate standard of safety and an agreed efficacy based on reported clinical trials and recorded clinical experience, the third category are traditional use HMPs, and they may be approved for marketing if they have been in use for at least 30 years, including 15 years in the European Union, and the therapeutic indication is considered safe for use without supervision [
5].
The basis of this work is to establish a traditional phytomedicine for intestinal comfort based on the European pharmacopeia. The phytomedicine will be a mixture of three plants (Myrtus communis L., Pimpinella anisum L., and Carum carvi L.). the mixture of those plants will be optimized with a mixture design experiment revealing the best ratio having the most potent activity basing on an in vivo pharmacological test (the acetic acid induced writhing test is used to assess the analgesic effect.).
P. anisum (aniseed is the common name of its fruit) traditional use is recognized by the European medicine agency (EMA/305345/2016) for indigestion complaints. C. carvi (caraway fruit is the common name for its fruit) is cultivated or gathered to obtain the fruit for medicinal use, it traditional use is recognized by the European medicine agency (EMA/498568/2015) for the relief of problems of the digestive system such as bloating and flatulence. M. communis (Leaves) traditional use is recognized by the French pharmacopeia (Liste A of the ANSM) as used in traditional European and overseas medicine. Although clinical studies provide limited data, the efficacy of these herbal medications seems conceivable, and there is evidence that they have been used safely in this manner for at least 30 years (including at least 15 years within the EU). Furthermore, the intended use of such plants is not subject to medical monitoring.
In order to elaborate a phytomedicine constituted of the combination of those three plants and instead of the trial-and-error method and to optimize the best combinations effectively, a mixture design is used. It is a class of response surface experiments that can be used to calculate the most efficient ratio of each component in a combination [
6].
2. Materials and Methods
2.1. Plant Material
Myrtus communis L. leaves, Pimpinella anisum L. seeds, and Carum carvi L. fruit were purchased for a local herbalist they were after that identified and authenticated by professor Anima Bari (Botanist), and a voucher specimen (Myrtus communis: BPRN60; Pimpinella anisum: BPRN73; Carum carvi: BPRN15) was deposited in the herbarium of the LBEAS laboratory.
2.2. Animals
For this research, Swiss albino mice weighing 20–25 g (both sexes) were utilized. The mice were acclimatized 1 week before the research and maintained under temperature (22 ± 1 °C), light (12:12 cycle) and humidity (45–50%), with unrestricted access to water and rodent chows [
7].
2.3. Preparation of the Extracts
The seeds of Pimpinella anisum, fruit of Carum carvi and the leaves of Myrtus communis were cleaned with tap water, dried at room temperature and reduced to a coarse powder with an electric grinder. The seeds were defatted before staring the extraction because of their high content in oil 10 g of powdered seeds washed out with 40 mL hexane three times.
The extraction of the plants starts with the use of ultrasound-assisted extraction apparatus, 10 g of defatted powder mixed with 70% ethanol for 40 min at a frequency of 35 KHz. Filtration of the extract occurred after that using Whatman filter paper No. 5. And with the help of a reduced pressure rotary evaporator, the filtrate was concentrated to dryness (crude extract and stored at 4 ºC until future use.
2.4. Plant Mixture Analysis
The extract mixture (3 mg) was derived by adding 200 μL of
N-methyl-
N trimethylsilyl trifluoroacetamide (MSTFA), then heated to 37 °C for 30 min. Then, 0.1 μL of this extract was injected for analysis [
8].
The analysis was conducted by using a gas chromatograph coupled with a mass spectrophotometer (GC-MS) of Brand Agilent Technologies Model 5973 with an Agilent column 19091S-433 HP-5MS, 30 m long, 0.25 mm inside diameter and 0.25 μm film thickness of the stationary phase. Helium was used as a carrier gas with a typical pressure range (psi) of 0.9 mL/sec. The oven temperature program was 60–300 °C at 10 °C/min and maintained at 300 °C for 20 min. The injector temperature was set to 250 °C, and the detector temperature to 260 °C. The extract (3 mg) is derived by adding 200 μL of N-methyl-N trimethylsilyl trifluoroacetamide (MSTFA), then heated to 37 °C for 30 min. Then, 0.1 μL of this extract was injected for analysis. The injection was carried out in split mode. The identification of the silylated compounds was possible by comparing the retention times with those of the standards and the spectral data obtained from the databases.
2.5. Experiment Design
The acetic acid-induced writhing test was used to evaluate the single and combined effects of the three plants, and the combination ratio was calculated using a mixture design experiment based on a simplex-centroid design matrix.
2.5.1. Mixture Design
Mixture designs are used to study the response changes according to the relative proportions of the components [
9]. Simplex-centroid design model was used to study the ternary effect of the plants. The distribution and repartition of the component mixtures varied from 1 (100% of that component) to 0 (0% of that component) represented as an equilateral triangle that includes key points (
Figure 1).
The main points 1,2,3 are 1st-degree centroids at the vertices of the equilateral triangle; each of them includes solely pure products. The binary mixes are represented by the sides of the triangle, and they are second-degree centroids. Point 4 is a third-degree centroid with the same value for all three components.
Table 1 describes the experimental design consisting of 10 experiments. Seven experiments consist of the seven points of the equilateral triangle, two experiments for the positive and one for negative control. All experiments consisted of five replications each.
Subsequently, data were fitted to a special cubic polynomial model using the least square regression for estimating unknown coefficients in the equation.
Given that Y is the desired response, bi is the magnitude of the effect of each single component, bij is the two components magnitude, and bijk is the three components magnitude. Xi indicates the proportions of the component (i) of the mixture.
JMP® Pro software version 13.0.0 for Windows was used for this study.
2.5.2. Acetic Acid-Induced Writhing in Mice
Mice were grouped (
n = 5), and the writhing test was performed a modified protocol from [
10]. The negative control consisted of a group of mice with induced writhing non treated, and the positive control consisted of three groups with three different treatments, the first group was treated with kalmagaz (600 mg/kg) which is phytomedicine based on vegetable charcoal and essential oils of mint and fennel prescribed for the elimination of intestinal gas, to improve digestion as well as intestinal comfort. The second positive control group was treated with spasfon (100 mg/kg) composed of phloroglucinol and trimethyl phloroglucinol prescribed for digestive tract pain, while the third positive control consisted of codoliprane (600 mg/kg) a potent analgesic drug composed of paracetamol and codeine. Seven other groups were treated with a different combination of the plant extract following the mixture design points (
Table 1).
The different treatments were administered orally to each animal 1 h before the induction of writing by intraperitoneal injection of 0.6% acetic acid in 0.9% normal saline, afterwards, the mice were observed and the number of abdominal constrictions and stretching were counted for a period of 30 min.
The percentage of protection against acetic acid was calculated using the following formula:
where Nc is the number of writhing in control, and Nt is the number of writhings in test animals.
2.5.3. Responses, Responses Goal and Optimum Formulation
The Response studied was the number of writhing in each test group following the different set of combination, the response goal is to have the minimum writhing during the 30 min of the test, and the optimum formulation will be calculated and deducted from the mixture design software taking into consideration the different responses.
2.6. Statistical Analysis
Graph Pad Prism version 7.0 for Windows was used for statistical analysis. A one-way analysis of variance was used to determine the difference between groups (ANOVA). All data are given as mean standard deviation.
3. Results and Discussion
3.1. Acetic Acid Induced Writhing Test
The results indicate that the different treatments significantly reduced the writhing number as compared to negative control mice (
p ≤ 0.001) (
Table 1). For the positive controls, spasfon percentage of inhibition of writhing was 48 % against 32% marked with Kalmagaz, while the best inhibition was for codoliprane with 85% other plant treatment marked reductions ranging between 65% and 76%.
The best percentage of inhibition of writhing for a single plant treatment were those of PA and MC with 73% then CC with 65%, and following those result, the best binary inhibition was the combination between PA and MC with 76%, followed by the combination between PA and CC with 71% and then MC and CC with 69%. The ternary combination of the three-plants also showed a perfect percentage of inhibition of writhing with 74%.
The result of the binary and ternary combination indicates that the plant mixture works on synergy and it was no antagonism as the effect remain clearly powerful with slight difference noted. Compared to positive control and taking into consideration the well-known effect of the three medicines, it is evident that those plants have an undeniable analgesic effect, for their single, binary and ternary combination.
3.2. Mixture Design
The optimal formulation is calculated by entering different results of the test into the mixture design software, setting the desired effect as a formulation compromising the different plant extract with the less writhing effect during the acetic acid-induced writhing test.
The response equation based on the most significant terms which represent the effects of the pure components (B1, B2, B3) is:
3.2.1. Ternary Plot and Mixture Profiler
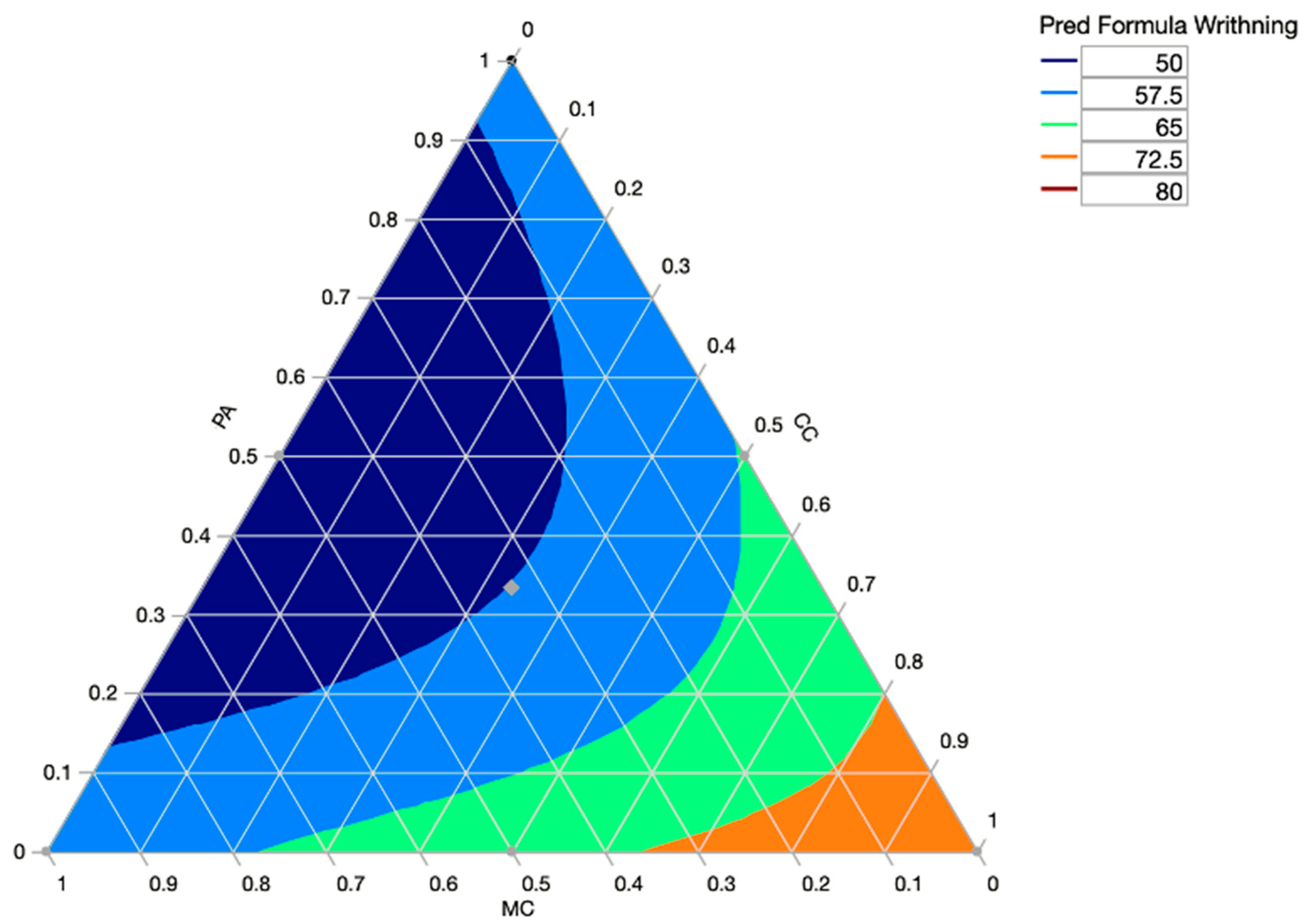
Figure 2 shows a ternary plot, which depicts the distribution and variability of three-part compositional data. It is a triangle with sides scaled from 0 to 1. Each of the three components is represented by one of the sides. A point is plotted in such a way that a line drawn perpendicularly from the point to each leg of the triangle intersects at the point’s component values.
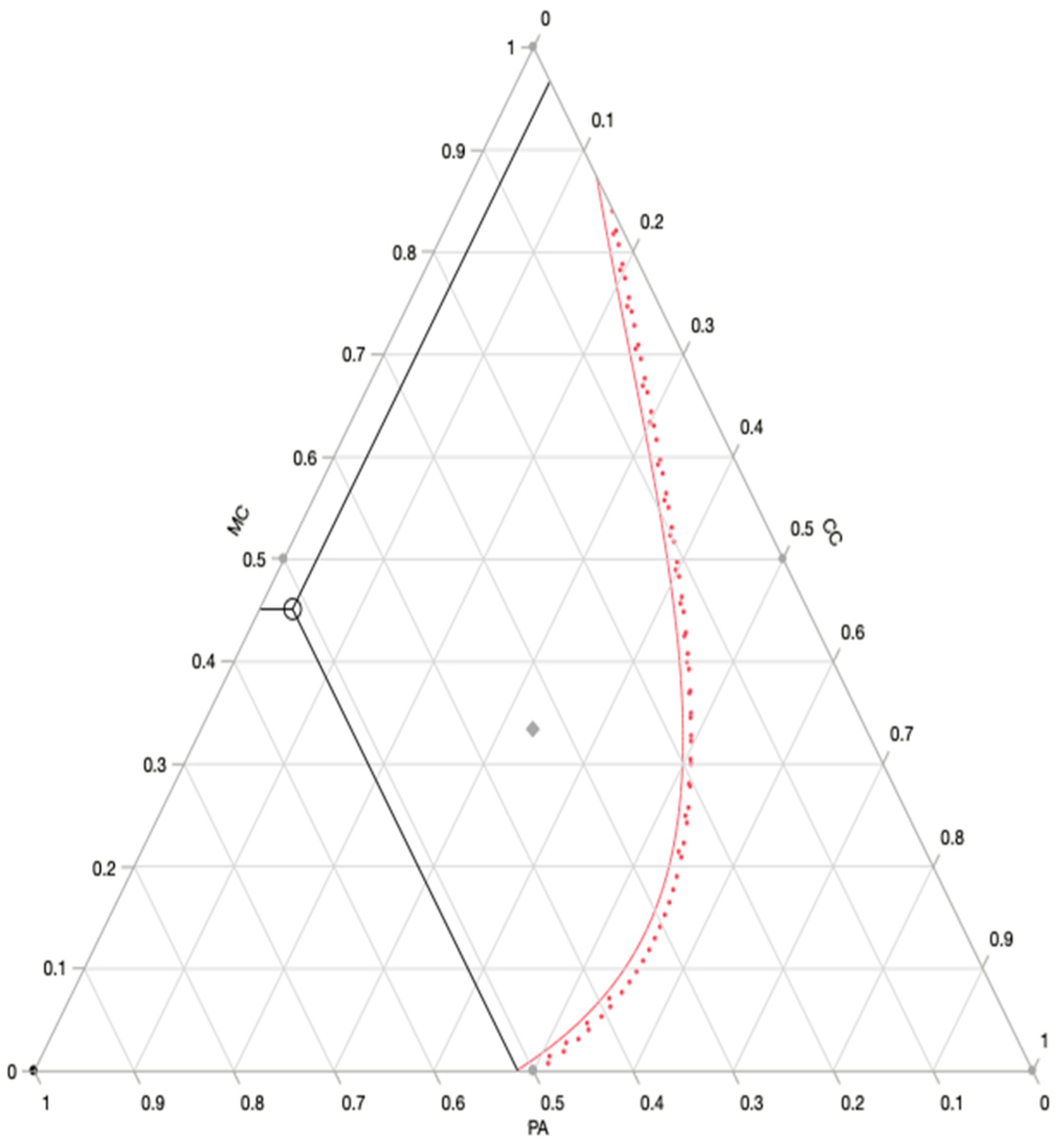
3.2.2. Optimal Formulation Prediction
The prediction profiler (
Figure 3) revealed the optimum combination, which was a ternary combination of
M. communis (MC),
P. anisum (PA) and
C. carvi (CC) with 45%, 52% and 3%, respectively.
For this ternary combination, the predicted value for our response “writhing number” was: 53 writhings in the 30 min of the test. Those results were obtained with the desirability of 93%. Furthermore, the mixture profiler (
Figure 4) visualized and optimized the response surfaces of the experiment where we show the response contours of the mixture experiment models on a ternary plot.
3.2.3. Test of the Optimum Formulation
The optimal formulation was tested following the same protocol of the acetic-acid induced writhing test. The number of writhing obtained was 48 ± 3,73 (n = 5) with an inhibition rate of 79%, the best reduction among the other combinations, confirming the prediction obtained by the software.
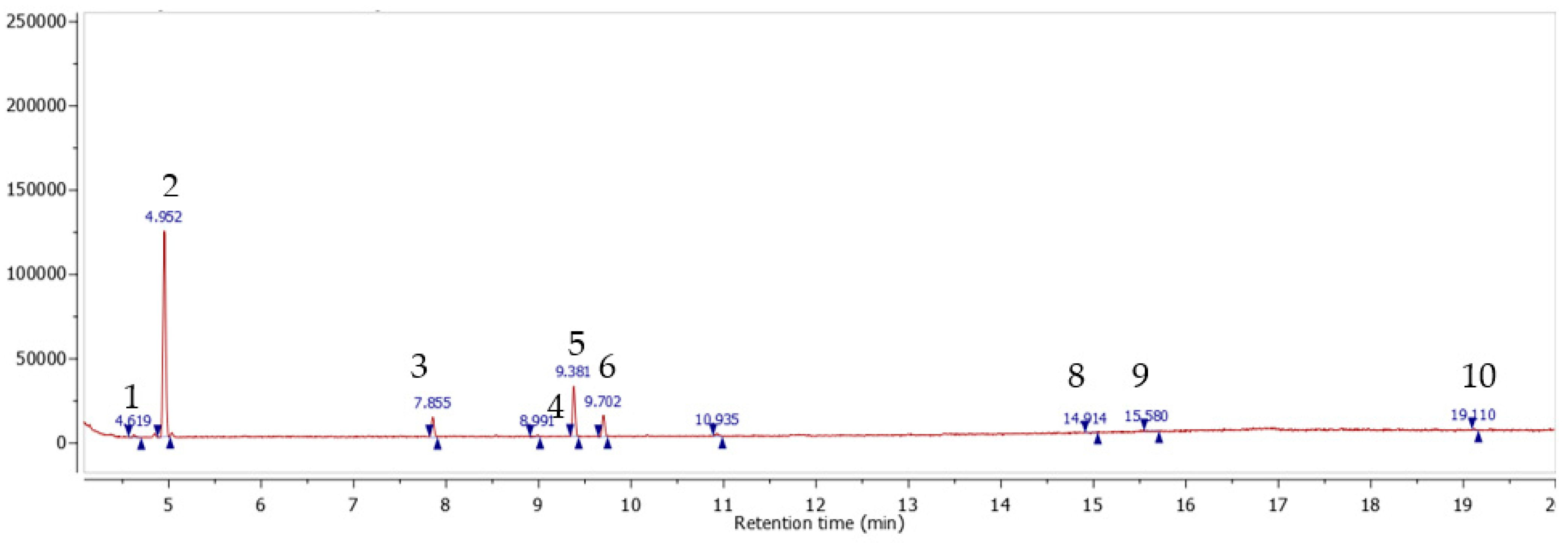
3.3. Composition Identification
The mixture composition was analyzed through a GC/MC method dedicated to polar compounds identification the analysis revealed the presence of 10 compounds: (1) syringic acid; (2) coumarin; (3) gallic acid; (4) protocatechuic acid; (5) hexadecenoic acid; (6) kaempferol; (7) caffeic acid; (8) oleic acid; (9)
trans-sesquisabinene hydrate; (10) cinnamic acid (
Figure 5 and
Table 2). Most of them are compounds known for their analgesic, antinociceptive and anti-inflammatory activities: syringic acid [
11], coumarin [
12], gallic acid [
13], protocatechuic acid [
14], kaempferol [
15] caffeic acid [
16].
Park et al. studied the antinociceptive potential of coumarin in mice, and they found that oral administration of coumarin at doses from 1 to 10 mg/kg demonstrated an antinociceptive effect in a dose-dependent manner [
17]. Caffeic acid was shown to possess antioxidant activity as well as a potential to inhibit 5-lipoxygenase (5-LOX) as part of its anti-inflammatory activity [
18]. In another study conducted by Mard et al. gallic acid demonstrated an ability to reduce the total area of gastric lesions after ischemia-reperfusion injury in rats [
19], and because of its strong inhibitory action in the enzyme kinetics study of Phospholipases A2, n-hexadecenoic acid may serve as an anti-inflammatory molecule [
20] same as kaempferol which was shown that it decrease the release of pro-inflammatory cytokines associated with the NFκB signaling pathway [
21,
22].
The well-studied bioactivity of the molecules explains the reason of the excellent activity of the extract clearly.
4. Conclusions
In this modest work, a phytomedicine for intestinal comfort was made based on the traditional use of the three plants inside the EU. With a documented base about their safety and their efficacy, this study was made to measure their power and formulate the most potent and synergic combination using mixture design experiment.
The optimum mixture of M. communis (45%), P. anisum (52%) and C. carvi (3%) was tested separately, and the results confirmed the accuracy and efficacy of this mixture mathematically obtained. In order to further exploit those results and others, the code concerning the quality of the plant cultivation and production must be followed and respected in order to obtain an authorization to possibly market any phytomedicine. Nowadays, due to the trust in well designed and approved medicines even if they were plant based, we consider this study as a model to be followed in order to valorize the scientific knowledge about medicinal plants to elaborate well-designed phytomedicines.
Author Contributions
Conceptualization, D.B. and I.E.-s.; software, H.M.; methodology, H.M.; formal analysis, A.A.; data curation, F.Z.J.; writing—original draft preparation, I.E.-s.; supervision, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of The Faculty of Sciences Dhar EL Mahraz Fez (12/2019/LBEAS #26/12/2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramawat, K.G.; Dass, S.; Mathur, M. The Chemical Diversity of Bioactive Molecules and Therapeutic Potential of Medicinal Plants. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 7–32. ISBN 978-3-540-79115-7. [Google Scholar]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Jawhari, F.Z.; Bari, A.; Cerruti, P.; Avella, M.; Andriy, A.; Andriy, D. Medicinal Plants Used to Treat Acute Digestive System Problems in the Region of Fez-Meknes in Morocco: An Ethnopharmacological Survey. Ethnobot. Res. Appl. 2020, 20. [Google Scholar] [CrossRef]
- Vlietinck, A.; Pieters, L.; Apers, S. Legal Requirements for the Quality of Herbal Substances and Herbal Preparations for the Manufacturing of Herbal Medicinal Products in the European Union. Planta Med. 2009, 75, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.R.; Van Staden, J. Smokescreens and Mirrors in Safety and Quality of Herbal Medicines: A Case of Commercialized Herbal Preparations. S. Afr. J. Bot. 2012, 82, 4–10. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and the Council of the European Union Directive 2004/ 24/EC of the European Parliament and of the Council of 31 March 2004 Amending, as Regards Traditional Herbal Medicinal Products, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use; European Parliament: Brussels, Belgium, 2004.
- Mechchate, H.; Ouedrhiri, W.; Es-safi, I.; Amaghnouje, A.; Jawhari, F.Z.; Bousta, D. Optimization of a New Antihyperglycemic Formulation Using a Mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea Var. Sylvestris Flavonoids: A Mixture Design Approach. Biologics 2021, 1, 154–163. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. In The National Academies Collection: Reports Funded by National Institutes of Health, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Kabran, G.R.; Mamyrbekova-Bekro, J.A.; Pirat, J.-L.; Bekro, Y.-A.; Sommerer, N.; Verbaere, A.; Meudec, E. Identification de Composés Phénoliques Extraits de Deux Plantes de La Pharmacopée Ivoirienne. J. De La Société Ouest-Afr. De Chim. 2014, 38, 57–63. [Google Scholar]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin:Optimization of a Novel Complete Antidiabetic Formulation Using a Mixture Design Approach. J. Nutr. Biochem. 2020, 88, 108520. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.-L.; Zhai, X.-F.; Zheng, X.; Zhang, L.; Wang, Y.-L.; Wang, Z. Anti-Inflammatory and Analgesic Activity of Total Flavone of Cunninghamia Lanceolata. Molecules 2012, 17, 8842–8850. [Google Scholar] [PubMed] [Green Version]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.S.; Taveira, M.L.; Viana, G.S.B.; Matos, F.J.A. Analgesic and Antiinflammatory Activities of Justicia Pectoralis Jacq and Its Main Constituents: Coumarin and Umbelliferone. Phytother. Res. 1997, 11, 211–215. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Lende, A.B.; Kshirsagar, A.D.; Deshpande, A.D.; Muley, M.M.; Patil, R.R.; Bafna, P.A.; Naik, S.R. Anti-Inflammatory and Analgesic Activity of Protocatechuic Acid in Rats and Mice. Inflammopharmacol 2011, 19, 255–263. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Gamaro, G.D.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of Rosmarinic and Caffeic Acids on Inflammatory and Nociception Process in Rats. ISRN Pharmacol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Sim, Y.-B.; Kang, Y.-J.; Kim, S.-S.; Kim, C.-H.; Kim, S.-J.; Lim, S.-M.; Suh, H.-W. Antinociceptive Profiles and Mechanisms of Orally Administered Coumarin in Mice. Biol. Pharm. Bull. 2013, 36, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.-M.; Kim, I.-T.; Park, Y.-M.; Ha, J.; Choi, J.-W.; Park, H.-J.; Lee, Y.S.; Lee, K.-T. Anti-Inflammatory Effect of Caffeic Acid Methyl Ester and Its Mode of Action through the Inhibition of Prostaglandin E2, Nitric Oxide and Tumor Necrosis Factor-α Production. Biochem. Pharmacol. 2004, 68, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Mard, S.A.; Mojadami, S.; Farbood, Y.; Naseri, M.K.G. The Anti-Inflammatory and Anti-Apoptotic Effects of Gallic Acid against Mucosal Inflammation-and Erosions-Induced by Gastric Ischemia-Reperfusion in Rats. In Proceedings of the Veterinary Research Forum, Urmia, Iran, 15 December 2015; Faculty of Veterinary Medicine, Urmia University; Volume 6, p. 305. [Google Scholar]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory Property of N-hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [PubMed]
- Suchal, K.; Malik, S.; Gamad, N.; Malhotra, R.K.; Goyal, S.N.; Chaudhary, U.; Bhatia, J.; Ojha, S.; Arya, D.S. Kaempferol Attenuates Myocardial Ischemic Injury via Inhibition of MAPK Signaling Pathway in Experimental Model of Myocardial Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Hou, J.; Zheng, Y.; Lin, C.; Ren, J. Protective Effect of Kaempferol on LPS plus ATP-Induced Inflammatory Response in Cardiac Fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).