COVID-19 Vaccinated Individuals Can Be a Source of SARS-CoV-2 Transmission—A Systematic Review
Abstract
:1. Introduction
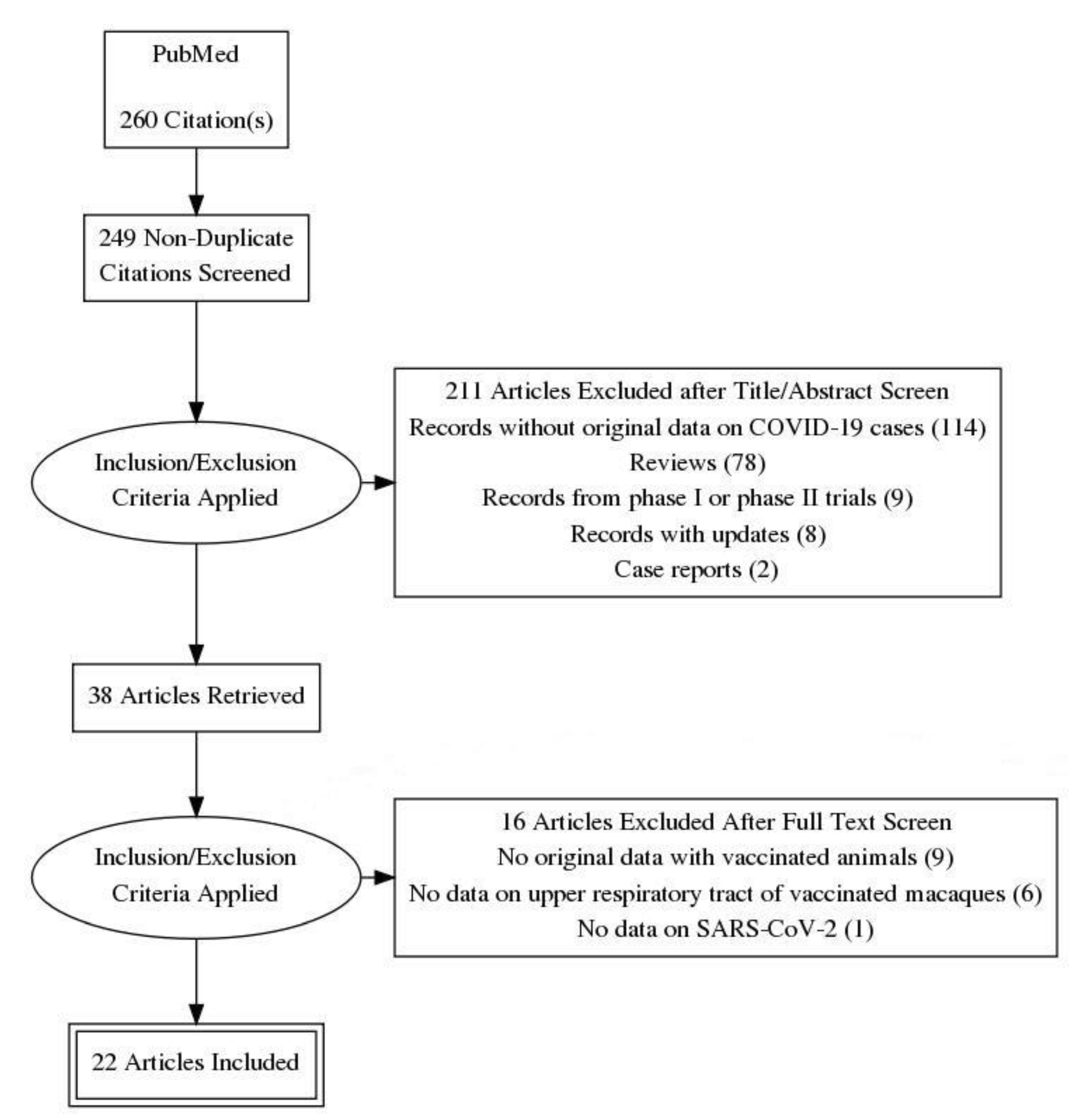
2. Materials and Methods
3. Results
3.1. Symptomatic Vaccinated COVID-19 Cases as a Possible Source
3.2. Asymptomatic Vaccinated COVID-19 Cases as a Possible Source
3.3. Viral Clearance in Vaccinated and Artificially Infected Macaques
3.3.1. Genomic RNA of SARS-CoV-2
3.3.2. Subgenomic RNA of SARS-CoV-2
3.3.3. Infectious SARS-CoV-2
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, S.; Ruktanonchai, N.W.; Zhou, L.; Prosper, O.; Luo, W.; Floyd, J.R.; Wesolowski, A.; Santillana, M.; Zhang, C.; Du, X.; et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature 2020, 585, 410–413. [Google Scholar] [CrossRef] [PubMed]
- WHO. Covid-19 Advice for the Public: Getting Vaccinated (8 April 2021). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 7 May 2021).
- Anonymous. Lambrecht: Geimpften Grundrechte Zurückgeben (22 January 2021). Available online: https://www.zdf.de/nachrichten/politik/corona-lambrecht-einschraenkungen-geimpfte-aufheben-100.html (accessed on 23 January 2021).
- Anonymous. Spahn für Mehr Freiheiten für Geimpfte Beim Reisen und Einkaufen (4 April 2021). Available online: https://www.aerzteblatt.de/nachrichten/122648/Spahn-fuer-mehr-Freiheiten-fuer-Geimpfte-beim-Reisen-und-Einkaufen (accessed on 6 April 2021).
- Dodd, R.H.; Pickles, K.; Nickel, B.; Cvejic, E.; Ayre, J.; Batcup, C.; Bonner, C.; Copp, T.; Cornell, S.; Dakin, T.; et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect. Dis. 2021, 21, 161–163. [Google Scholar] [CrossRef]
- Costantino, C.; Mazzucco, W.; Azzolini, E.; Baldini, C.; Bergomi, M.; Biafiore, A.D.; Bianco, M.; Borsari, L.; Cacciari, P.; Cadeddu, C.; et al. Influenza vaccination coverage among medical residents: An Italian multicenter survey. Hum. Vaccines Immunother. 2014, 10, 1204–1210. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020-March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, B.; Ganatra, S.R.; Gazi, M.; Cole, J.; Thippeshappa, R.; Alfson, K.J.; Clemmons, E.; Gonzalez, O.; Escobedo, R.; et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 2021, 6, 73–86. [Google Scholar] [CrossRef]
- Yu, P.; Qi, F.; Xu, Y.; Li, F.; Liu, P.; Liu, J.; Bao, L.; Deng, W.; Gao, H.; Xiang, Z.; et al. Age-related rhesus macaque models of COVID-19. Anim. Models Exp. Med. 2020, 3, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Yao, Y.F.; Yang, X.L.; Zhou, Y.W.; Gao, G.; Peng, Y.; Yang, L.; Hu, X.; Xiong, J.; Jiang, R.D.; et al. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res. 2020, 30, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, H.; Guo, L.; Liang, Y.; Li, J.; Wang, X.; Hu, Y.; Wang, L.; Liao, Y.; Yang, F.; et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: A nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020, 16, e1008949. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.; Schulz, J.; Holbrook, M.; Bushmaker, T.; Carmody, A.; Port, J.; Yinda, K.C.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv 2021. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Grobben, M.; Claireaux, M.; de Gast, M.; Marlin, R.; Chesnais, V.; Diry, S.; et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 2021, 184, 1188–1200. [Google Scholar] [CrossRef]
- Yadav, P.D.; Ella, R.; Kumar, S.; Patil, D.R.; Mohandas, S.; Shete, A.M.; Vadrevu, K.M.; Bhati, G.; Sapkal, G.; Kaushal, H.; et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021, 12, 1386. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721. [Google Scholar] [CrossRef]
- Sanchez-Felipe, L.; Vercruysse, T.; Sharma, S.; Ma, J.; Lemmens, V.; Van Looveren, D.; Arkalagud Javarappa, M.P.; Boudewijns, R.; Malengier-Devlies, B.; Liesenborghs, L.; et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 2021, 590, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 100230. [Google Scholar] [CrossRef] [PubMed]
- Dagotto, G.; Mercado, N.B.; Martinez, D.R.; Hou, Y.J.; Nkolola, J.P.; Carnahan, R.H.; Crowe, J.E., Jr.; Baric, R.S.; Barouch, D.H. Comparison of Subgenomic and Total RNA in SARS-CoV-2 Challenged Rhesus Macaques. J. Virol. 2021, 95, 8. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S.; et al. Low-Dose Ad26.COV2.S Protection Against SARS-CoV-2 Challenge in Rhesus Macaques. bioRxiv 2021. [Google Scholar] [CrossRef]
- Routhu, N.K.; Cheedarla, N.; Gangadhara, S.; Bollimpelli, V.S.; Boddapati, A.K.; Shiferaw, A.; Rahman, S.A.; Sahoo, A.; Edara, V.V.; Lai, L.; et al. A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity 2021, 54, 542–556. [Google Scholar] [CrossRef]
- Guebre-Xabier, M.; Patel, N.; Tian, J.H.; Zhou, B.; Maciejewski, S.; Lam, K.; Portnoff, A.D.; Massare, M.J.; Frieman, M.B.; Piedra, P.A.; et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 2020, 38, 7892–7896. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Ehaideb, S.N.; Abdullah, M.L.; Abuyassin, B.; Bouchama, A. Evidence of a wide gap between COVID-19 in humans and animal models: A systematic review. Crit. Care 2020, 24, 594. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J.; Nixon, D.F.; Moore, J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Geimpfter Soll Mehrere Personen in Klinik Angesteckt Haben (2 April 2021). Available online: https://www.rtl.de/cms/corona-alarm-in-halle-geimpfter-soll-unwissentlich-mehrere-personen-angesteckt-haben-4732838.html (accessed on 6 April 2021).
- Hall, M.A.; Studdert, D.M. Privileges and Immunity Certification during the COVID-19 Pandemic. JAMA 2020, 323, 2243–2244. [Google Scholar] [CrossRef] [PubMed]
| Vaccine (Manufacturer) | Symptomatic COVID-19 Cases * Among | Reference | |
|---|---|---|---|
| Vaccinated Subjects | Control Subjects | ||
| AZD1222 (AstraZeneca) | 74 of 7.201 (1.0%) | 197 of 7.178 (2.7%) | [7] |
| mRNA 1273 (Moderna) | 11 of 15.210 (0.07%) | 185 of 15.210 (1.2%) | [8] |
| BNT162b2 (BioNTech) | 8 of 18.198 (0.04%) | 162 of 18.325 (0.9%) | [9] |
| Sputnik V (Gamaleya) | 16 of 14.964 (0.1%) | 62 of 4.902 (1.3%) | [10] |
| Vaccine (Manufacturer) | Animals | Timing of SARS-CoV-2 Challenge | Nasal SARS-CoV-2 Challenge | SARS-CoV-2 Challenge in Other Organs | Effect in Control Animals | Effect in Vaccinated Animals | Reference |
|---|---|---|---|---|---|---|---|
| AZD1222 (AstraZeneca) | 6 per group | 2 weeks after the second intramuscular vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on days 0 and 28 *** | 4 × 105 * | Intratracheal: 1.6 × 106 * Oral: 4 × 105 * Ocular: 2 × 105 * | Approx. 106 cpm on day 1, between 102 and 104 cpm on day 7 | Approx. 105 to 107 cpm on day 1, between 102 and 105 cpm on day 7; no significant difference to control group | [16] |
| AZD1222 (AstraZeneca) | 4 per group | 56 days after the intranasal vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on day 0 | 2 × 105 * | Intratracheal: 8 × 105 * | Between 103 and 108 cpm on day 3, between 103 and 104 cpm on day 7 | Between 103 and 106 cpm on day 3, below limit of detection (103) on day 7; no significant difference to control group | [17] |
| BNT162b2 (BioNTech Pfizer) | 9 (control group) and 6 (vaccine group) | 55 days after the second intramuscular vaccination with 100 µg on days 0 and 21 **** | 6.3 × 105 ** | Intratracheal: 6.3 × 105 ** | Between 103 and 105 viral RNA copies on days 1, 3 and 6 | Between 103 and 106 viral RNA copies on day 1, below limit of detection (103) on days 3 and 6 (no comparative statistics) | [18] |
| SARSCoV-2 S-I53-50NP (not described) | 4 or 6 per group | 2 weeks after the third vaccination with 50 µg on days 0, 28 and 70 | 105 ** | Intratracheal: 5.9 × 105 ** | Approx. 108 cpm on day 2, mostly none detected after day 8 | Approx. 105 cpm on day 2, none detected after day 3 | [19] |
| BBV152 (Bharat Biotech) | 5 per group | 4 weeks after the second intramuscular vaccination with 3 µg vaccine and adjuvant-B on days 0 and 14 | 106 * | Intratracheal: 106.5 * | Approx. 107 cpm on days 1 and 3, between 104 and 105 cpm on days 5 and 7 | Approx. 105 cpm on day 1, no RNA detected on days 3, 5 and 7 | [20] |
| Six different DNA vaccines (Janssen) | 4 or 5 per group | 3 weeks after the second intramuscular vaccination with a vaccine on days 0 and 21 | 1.1 × 104 ** | Intratracheal: 1.1 × 104 ** | Approx. 107 cps on day 2, slowly going down to 102 cps on day 14 | Between 103 and 108 cps on day 2, mostly going down to less than 102 cps on day 10 | [21] |
| RBD ***** (not described) | 3 or 4 per group | 3 weeks after the second intramuscular vaccination with 20 or 40 µg on days 0 and 7 | 5 × 105 ** | None | Between 104 and 106 cpm on days 2–6 | Between 1 and 106 cpm on days 2–6 | [22] |
| BBIBP-CorV *** (not described) | 2 or 4 per group | 10 days after the second intramuscular vaccination with 2 or 8 µg on days 0 and 14 | None | Intratracheal: 106 * | Between 105 and 106 cpm on days 3, 5 and 7 | Approx. 103 cpm on day 3, between 102 and 105 on day 5, between 0 and 102 on day 7 | [23] |
| YF17D ****** (not described) | 6 per group | 21 days after the second subcutaneously vaccination with 105 PFU on days 0 and 7 | 7.5 × 103 * | Intratracheal: 7.5 × 103 * | Between 102 and 106 cpm on days 1–4 | Mostly < 102 cpm on days 1–4 | [24] |
| ChAd-SARS-CoV-2-S (not described) | 6 per group | 4 weeks after the intranasal vaccination with 1011 viral particles | 5.7 × 105 * | Intratracheal: 5.7 × 105 * | Between 103 and 104 cpm on day 1, slowly going down to 0 to 103 on day 7 | Between 102 and 103 cpm on day 1, slowly going down to 0 to 10 on day 7 | [25] |
| Vaccine (Manufacturer) | Animals | Timing of SARS-CoV-2 Challenge | Nasal SARS-CoV-2 Challenge (PFU) | SARS-CoV-2 Challenge in Other Organs (PFU) | Effect in Control Animals | Effect in Vaccinated Animals | Reference |
|---|---|---|---|---|---|---|---|
| AZD1222 (AstraZeneca) | 6 per group | 2 weeks after the second intramuscular vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on days 0 and 28 *** | 4 × 105 * | Intratracheal: 1.6 × 106 * Oral: 4 × 105 * Ocular: 2 × 105 * | Between 102 and 105 cpm on day 1, below limit of detection (102) on day 7 | Between 102 and 105 cpm on day 1, below limit of detection (102) on day 7; no significant difference to control group | [16] |
| AZD1222 (AstraZeneca) | 4 per group | 56 days after the intranasal vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on day 0 | 2 × 105 * | Intratracheal: 8 × 105 * | Between 102.5 and 104.5 cpm on day 1, between 102.5 and 103.5 cpm on day 5, below limit of detection (102.5) on day 7 | Between 102.5 and 103.5 cpm on day 5, below limit of detection (102.5) on days 1, 3 and 7; no significant difference to control group | [17] |
| SARSCoV-2 S-I53-50NP (not described) | 4 or 6 per group | 2 weeks after the third vaccination with on days 0, 28 and 70 | 105 ** | Intratracheal: 5.9 × 105 ** | Between 105 and 107 cpm on day 2, between 102 and 104 cpm on days 5 and 6 | No sgRNA detected on days 2, 5 and 6 | [19] |
| BBV152 (Bharat Biotech) | 5 per group | 4 weeks after the second intramuscular vaccination with 3 µg vaccine and adjuvant-B on days 0 and 14 | 106 * | Intratracheal: 106.5 * | Approx. 107 cpm on days 1, 3 and 7 | No sgRNA detected on days 1, 3, 5 and 7 | [20] |
| Six different DNA vaccines (Janssen) | 4 or 5 per group | 3 weeks after the second intramuscular vaccination with a vaccine on days 0 and 21 | 1.1 × 104 ** | Intratracheal: 1.1 × 104 ** | Between 105 and 107 cps on days 1 to 4, slowly going down to 102 cps on day 14 | Between 102 and 105 cps on day 2, mostly going down to less than 102 cps on day 7 | [21] |
| Variant S.PP (Janssen) | 20 (control group) and 6 (vaccine group) | 6 weeks after the intramuscular vaccination with 1011 viral particles on day 0 | 5.5 × 103 ** | Intratracheal: 5.5 × 103 ** | Between 104 and 108 viral copies on day 2, between 101.5 and 105 on day 10 | Between 101.5 and 103.5 viral copies on day 1, below limit of detection on days 4 to 10 | [27] |
| mRNA-1273 (Moderna) | 8 per group | 4 weeks after the second intramuscular vaccination with 100 µg on days 0 and 28 **** | 1.9 × 105 ** | Intratracheal: 5.7 × 105 ** | Between 101.5 and 105.5 cpm on days 1 and 2, between 101.5 and 104.5 cpm on day 4, below limit of detection (101.5) on day 7 | Between 101.5 and 104.5 cpm on day 1, below limit of detection (101.5) on days 2, 4 and 7 (one animal with 102.5 cpm on day 4); peak levels on days 2–7: p = 0.009 | [28] |
| RBD (not described) | 3 or 4 per group | 3 weeks after the second intramuscular vaccination with 20 or 40 µg on days 0 and 7 | 5 × 105 ** | None | Between 103 and 105 cpm on days 2–6 | No sgRNA detected on days 2–6 | [22] |
| Vaccine Ad5-S-nb2 (Guangzhou nBiomed) | 2 or 4 per group | 30 days after an intramuscular vaccination (high dose or low dose) or an intranasal and oral application of the vaccine | None | Intratracheal: 2 × 104 or 400 * | Between 102 and 106 cpm on days 1–10 | Mostly less than 102 cpm on days 1–10, one peak of 104 cpm on day 6 (intranasal and oral application) | [29] |
| VX-CoV2327 vaccine (Novavax) | 4 per group | After the second intramuscular vaccination with 2.5, 5 or 25 µg on days 0 and 21 | 5.5 × 103 ** | Intratracheal: 5.5 × 103 ** | Between 10 and 104 cpm on days 2 and 4 | None detected | [32] |
| Ad26.COV2.S (Janssen) | 5 or 10 per group | 6 weeks after an intramuscular vaccination with 1 × 1011, 5 × 1010, 1.125 × 1010 or 2 × 109 viral particles on day 0 | 1.1 × 104 ** | Intratracheal: 1.1 × 104 ** | Peak values between 104 and 107 cps | Highest dose: none detected Middle doses: mostly none detected Lowest dose: mostly between 105 and 107 cps | [30] |
| MVA-based COVID-19 vaccine (not described) | 5 per group | 4 weeks after the second intramuscular vaccination with 108 PFU on weeks 0 and 4 | 2.5 × 104 ** | Intratracheal: 2.5 × 104 ** | Between 10 and 107 cpm on days 2, 4 and 7, none on day 10 | Between 10 and 105 cpm on days 2, 4 and 7, none on day 10 | [31] |
| ChAd-SARS-CoV-2-S (not described) | 6 per group | 4 weeks after the intranasal vaccination with 1011 viral particles | 5.7 × 105 * | Intratracheal: 5.7 × 105 * | Between 105 and 107 cpm on days 1–7 | Between 103 and 108 cpm on days 1–7 | [25] |
| Vaccine (Manufacturer) | Animals | Timing of SARS-CoV-2 Challenge | Nasal SARS-CoV-2 Challenge (PFU) | SARS-CoV-2 Challenge in Other Organs (PFU) | Effect in Control Animals | Effect in Vaccinated Animals | Reference |
|---|---|---|---|---|---|---|---|
| AZD1222 (AstraZeneca) | 6 per group | 2 weeks after the second intramuscular vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on days 0 and 28 * | 4 × 105 | Intratracheal: 1.6 × 106 Oral: 4 × 105 Ocular: 2 × 105 | Detected in 4 animals on day 1, in 1 animal on day 3, and in 0 animals on days 5 and 7 | Detected in 2 animals on day 1 and in 0 animals on days 3, 5 and 7 (no comparative statistics) | [16] |
| AZD1222 (AstraZeneca) | 4 per group | 56 days after the intranasal vaccination with 2.5 × 1010 ChAdOx1 nCoV-19 on day 0 | 2 × 105 | Intratracheal: 8 × 105 | 10 and 106 on day 1 | 10–150 on day 1 (p < 0.05) | [17] |
| Variant S.PP (Janssen) | 20 (control group) and 6 (vaccine group) | 6 weeks after the intramuscular vaccination with 1011 viral particles on day 0 | 5.5 × 103 | Intratracheal: 5.5 × 103 | Infectious virus on day 2 between 10 and 1000 PFU per swab | No infectious virus on day 2 | [27] |
| ChAd-SARS-CoV-2-S (not described) | 6 per group | 4 weeks after the intranasal vaccination with 1011 viral particles | 5.7 × 105 | Intratracheal: 5.7 × 105 | 4 animals with infectious virus on day 1 | 1 animal with infectious virus on day 1 | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampf, G. COVID-19 Vaccinated Individuals Can Be a Source of SARS-CoV-2 Transmission—A Systematic Review. Hygiene 2021, 1, 1-11. https://0-doi-org.brum.beds.ac.uk/10.3390/hygiene1010001
Kampf G. COVID-19 Vaccinated Individuals Can Be a Source of SARS-CoV-2 Transmission—A Systematic Review. Hygiene. 2021; 1(1):1-11. https://0-doi-org.brum.beds.ac.uk/10.3390/hygiene1010001
Chicago/Turabian StyleKampf, Günter. 2021. "COVID-19 Vaccinated Individuals Can Be a Source of SARS-CoV-2 Transmission—A Systematic Review" Hygiene 1, no. 1: 1-11. https://0-doi-org.brum.beds.ac.uk/10.3390/hygiene1010001




