Malignant and Non-malignant Atypical Circulating Cell in Cancer: Their Heterogeneity and Plasticity
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Metastasis".
Viewed by 28571Editors
Interests: cancer metastasis; circulating tumor cells; microenvironment; cancer heterogeneity and plasticity; biomarkers; resistance to treatment
Interests: Circulating Tumor Cells; Tumor Plasticity; Tumor Cell Cultures
Topical Collection Information
Dear Colleagues,
Solid tumors induce the presence of atypical cell types in the blood circulation. The ease of sampling these atypical circulating cells, although counterbalanced by their scarceness, holds the potential to help us better understand tumor evolution and drug resistance.
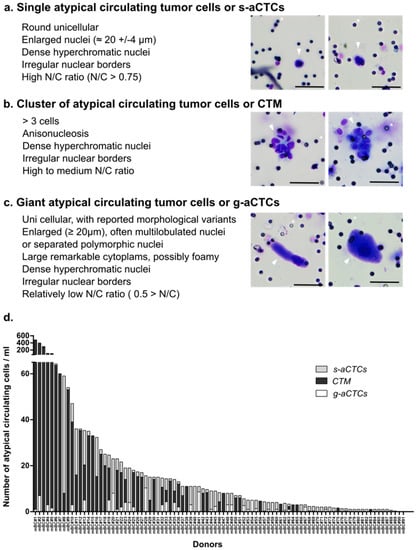
The most-described atypical circulating cells in cancers are called circulating tumor cells (CTCs). They can potentially generate distant metastasis, and many studies demonstrate their role in predicting clinical outcomes. The initial view that CTCs are defined by their epithelial phenotype has been challenged by the discovery that CTCs are a heterogeneous cell population characterized by plasticity and can also include cells that have lost some of their epithelial traits. Moreover, reports indicate that many other atypical circulating cells can be found in the blood of cancer patients, and, despite not being of epithelial origin and malignant, can play an important role in metastasis and hold strong prognostic value.
This Special Issue will focus on atypical circulating cells—of a malignant origin or not—found in the blood of cancer patients, and how their heterogeneity can provide us with a better understanding of tumor behavior. The aim of this Special Issue is to provide insights into the physical, biological, and chemical cues that generate this heterogeneity and trigger cell plasticity/reprogramming, such as adjustment to stressful conditions or changes favoring tumor progression.
We look forward to your contribution to this Special Issue.
Dr. Emilie Mamessier-Birnbaum
Dr. Claire Acquaviva
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- circulating tumor cells
- atypical circulating cells
- cellular plasticity
- cellular heterogeneity
- cell reprogramming
- epithelial-to-mesenchymal transition
- resistance to treatment
- physical cues
- stress
- anti-tumor immunity
- anoikis
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.
Title: Evaluation of a metabolism- related gene expression in Circulating Tumor Cellsfrom patients with early stage Non- Small Cell Lung Cancer
Authors: /
Affiliation: /
Abstract: Purpose: One of the key hallmarks of cancer is metabolic reprogramming and marked increase in the rate of glycolysis that contributeto rapid cell proliferation and growth. Cancer cells consume elevated levels of glucose and produce high amounts of lactate even in the presence of oxygen, a process known as aerobic glycolysis or the “Warburg Effect”. The “Warburg Effect” demonstrates the importance of glycolysis in the development of primary and metastatic cancers. In the present study, we examined the gene expression profile of three metabolism- related genes (MRGs): Hexokinase 2 (HK2), Phosphoglycerate Dehydrogenase (PHGDH) and Monocarboxylate Transporter 1 (MCT1) in circulating tumor cells (CTCs) derived from early stage Non-Small Cell Lung Cancer (NSCLC) patients. We further evaluated their expression in association with clinicopathological parameters and their prognostic significance. Experimental design: Highly sensitive RT- qPCR assays for the three MRGs were developed, analytically validated and then applied to study MRGs expression in CTC isolated through a size- dependent microfluidic device (Parsortix, ANGLE) from the peripheral blood of 46 patients among different timepoints: at baseline (pre- surgery), one month after surgery (corresponding samples)and at the time of relapse. We also evaluated the MRG gene expression levels in ten fresh- frozen NSCLC tissues and their corresponding adjacent non- neoplastic tissues. Peripheral blood from 18 healthy donors (HD) were analyzed in the exact same procedure for normalization. We further evaluated the association between Mesenchymal biomarkers (TWIST or Vimentin) and MRGs in the corresponding samples from previous studies. Results:HK2 and MCT1 were differentially expressed between HD and NSCLC patients at baseline. Overexpression of HK2 was detected in 14/ 46 (30,4%), 1/36 (2,8%) and 0/7 (0%) patients at baseline, one month later and at the time of relapse respectively. In the case of PHGDH overexpression was detected in 7/ 46 (15,2%) patients at baseline, 4/36 (11,1%) at one month later and 3/7 (42,8%) at the time of relapse. Lastly, MCT1 overexpression was detected in 15/46 (32,6%) patients at the baseline, 7/38 (18,4%) one month later and 2/9 (22,2%) at the time of relapse. Kaplan- Meier Analysis showed no correlation between the expression of MRGs in CTCs with progression- free survival. We observed that overexpression of Mesenchymal biomarkers was associated with higher expression levels of MCT1 and HK2. Conclusion:MRGs overexpression was observed at a high frequency in CTCs mainly at the baseline, while the different expression levels of HK2 and MCT1 between HD and NSCLC patients at the baseline supports their role in the cancer setting. We strongly believe that MRGs overexpression in CTCs merits further evaluation as a non- invasive circulating tumor biomarker in a large and well- defined cohort of NSCLC patients.
Title: Selective deregulation of circular RNAs by anticancer agents in breast cancer
Authors: Anna Terrazzan 1, Francesca Crudele 2, Fabio Corrà 1, Nicoletta Bianchi 1, ‡, * and Stefano Volinia 1,3, ‡, *
Affiliation: 1 University of Ferrara, Department of Translational Medicine, Ferrara, Italy; [email protected], [email protected], [email protected], [email protected] 2 Genetics Unit, Institute for Maternal and Child Health, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS) Burlo Garofolo, Trieste (TS), Italy; [email protected] 3 Laboratory for Advanced Therapy Technologies (LTTA), Ferrara, Italy; * Correspondence: NB, [email protected]; Tel.: +39-0532-455447 and SV, [email protected]; Tel.: +39- Tel.: +39- 0532-455714
Abstract: Abstract: Altered expression of circular RNAs has been described in breast cancer, but less well in its relation with that of the cognate linear transcripts. Furthermore, not much is known about the effect of cancer drugs on circular RNA regulation. Thus, we investigated the dysregulation of 12 cancer related circRNAs and that of their host genes, in two breast cancer cell lines upon various drug treatments. We selected 14 well-known anticancer agents affecting different cellular pathways. We investigated the impact of each drug on cognate circRNA and linear RNA levels and described a range of outcomes. Summarizing, the circRNAs from MCF-7 and MDA-MB-231 cells, were differently affected by various cancer drugs. For examples, the VIRK1 RNA cognates were similarly affected in both cell lines by multiple drugs, while as IGF1R and NCOA3 circRNA and mRNA cognates dis-played a cell- and drug-dependent response, being both modulated in MCF-7 exclusively by AZD5363. In the Akt/PI3K signaling pathway, circHIPK3 was deregulated by BYL719, LEE011, AZD8055 in MCF-7, whereas in MDA-MB-231 cells linHIPK3 was modulated by AZD7762. Overall, our results underline how cancer drugs altered circRNA levels in specific manners, particularly when compared to the cognate messenger RNAs.
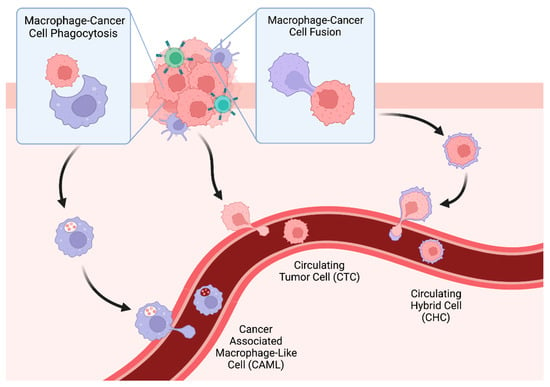
Title: Circulating Macrophage Like Cells in Patients with Non-metastatic Adenocarcinoma of the Esophagus – Cytomorphological Heterogeneity
Authors: Jasmina Kuvendjiska; Clara Braun; Birte Kulemann
Affiliation: Department of Surgery, University Medical Center of Schleswig-Holstein-Campus Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany
Abstract: Background: Esophageal adenocarcinoma often shows systemic recurrences despite being treated with curative intention. Liquid Biopsy promises insights into the metastatic process and thus improvements in oncological diagnostics and therapy. In addition to well-known circulating tumor cells, other tumor-associated cells originating from the tumor microenvironment have recently come into focus, for example Cancer-associated makrophage-like cells (CAML). They are believed to be representatives of the inflammatory tumor microenvironment shed into bloodstream. In this multi-centered study, we investigated presence and cytomorphological appearance of CAML in a large collective of patients with non-metastatic EAC in a pretherapeutic setting. Methods: Blood samples of 252 patients from centers all over Germany with clinically non-metastatic EAC were taken before starting curative treatment and were processed using ScreenCell filtration devices. Cytological analysis was performed in May-Grünwald-Giemsa staining. CAML were defined by their morphological characteristics. Correlation with presence and number of CAML and cTNM-status was statistically analyzed. In addition, immunofluorescence staining with the mesenchymal marker Vimentin was performed on a small study collective. Results: CAML were detected in 31,8% (n=80) of patients. They showed cytomorphological heterogeneity. Their presence and cell count did not significantly correlate with pretherapeutic cTNM. Even in patients with small tumor sizes and without lymph node infiltration, cell counts were high. CAML showed heterogenous staining patterns for Vimentin. Conclusions: This multicentered study shows presence and heterogeneity of CAML isolated by ScreenCell in patients with locally advanced EAC. As EAC is seen as paradigma of inflammation-induced cancers, findings of possible blood markers for the inflammatory tumor micorenvironment are of great interest.
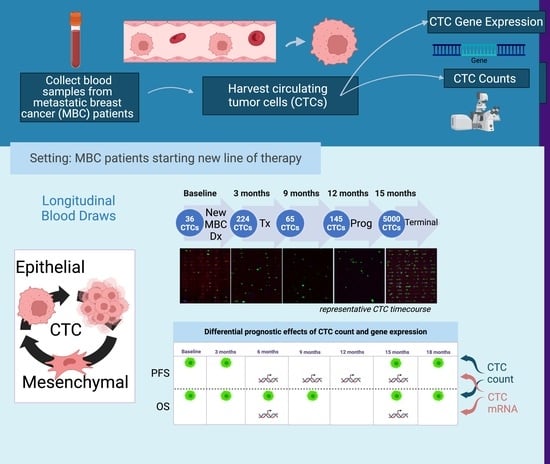
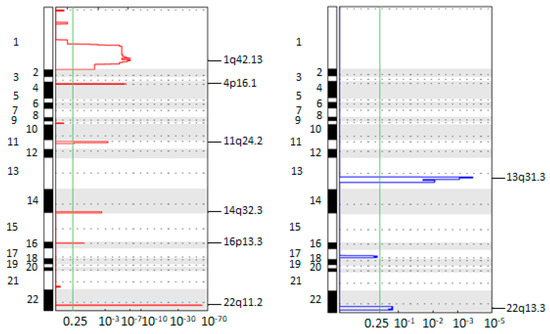
Title: Dissecting molecular heterogeneity of Circulating Tumor Cells (CTCs) from metastatic breast cancer patients through copy number aberration (CNA) and single nucleotide variant (SNV) single cell analysis
Authors: Rossi, Tania
Affiliation: Biosciences Laboratory, IRCCS, Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, 47014 Meldola, Italy
Abstract: Background: circulating tumor cells (CTCs) heterogeneity contributes to counteract their introduction in clinical practice. Through single-cell sequencing we aim at exploring CTC heterogeneity in metastatic breast cancer (MBC) patients. Methods: Single CTCs were isolated using DEPArray NxT. After whole genome amplification, libraries were prepared for copy number aberration (CNA) and single nucleotide variant (SNV) analysis and sequenced using Ion GeneStudio S5 and Illumina MiSeq, respectively. Results: CTCs demonstrate distinctive mutational signatures but retain molecular traces of their common origin. CNA profiling identifies frequent aberrations involving critical genes in pathogenesis: gains of 1q (CCND1) and 11q (WNT3A), loss of 22q (CHEK2). Longitudinal single-CTC analysis allows tracking of clonal selection and the emergence of resistance-associated aberrations, such as gain of a region in 12q (CDK4). A group composed by CTCs from different patients sharing common traits emerges. Further analyses identify losses of 15q and enrichment of term associated with pseudopodium formation as frequent and exclusive events. Conclusions: CTCs from MBC patients are heterogeneous, especially concerning their mutational status. Single-cell analysis allows the identification of aberrations associated with resistance, and is a candidate tool to better address treatment strategy. The translational significance of the group populated by similar CTCs should be elucidated.