Stemness and Drug-Persistence in Cancer
Share This Topical Collection
Editors
Topical Collection Information
Dear Colleagues,
Adult stem cells are fundamental for tissue homeostasis and repair in adult organisms. They are influenced by different pathways to maintain self-renewal and multipotency potential. In cancer, the constitutive activation of these specific pathways implicates the modulation of these stem cells and can sustain the long-term clonal maintenance of the neoplasm. The behavior and characterization of such cells and their plasticity are still not well understood. Tumor relapse that is responsible for most of the deaths of cancer patients has been linked to cancer stem cells that are not efficiently targeted by the current therapies. Advances in the last few years have demonstrated that a small pool of drug-tolerant cancer cells, not essentially always cancer stem cells, are responsible for treatment failure. Novel insights into the discovery of stem cell function and persistence in cancer provide promising directions for future cancer therapeutic applications.
The purpose of this Collection is to contribute to the definition of the stemness and drug-persistence properties in tumoral cells. Furthermore, by disentangling the heterogeneity within tumors, we aim to identify the key players and properties that enable cells to cause cancer growth and spreading; and to describe the mechanisms involved in cell plasticity to understand how cancer cells survive conventional therapies. The Collection will include molecular stem cell signatures, drug-resistance mechanisms, novel in vitro and in vivo models for studying stemness and studies that describe the potential applications of cells to cancer therapy. To this end, we are looking for experiments using mouse models, clinical association analyses, high-throughput approaches, next-generation sequencing, single cell technologies, patient-derived material and 3D organoids, which demonstrate intra-tumor heterogeneity. This Collection welcomes both reviews and original research articles.
Prof. Dr. Cinzia Allegrucci
Prof. Dr. Paloma Ordóñez-Morán
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- stem cells in cancer
- self-renewal and multipotency
- stem cell markers
- cell plasticity
- tumor heterogeneity
- drug resistance
- epigenetics in cancer
- tumor dormancy and relapse
- in vivo and in vitro models
- cancer genomics
Related Special Issue
Published Papers (7 papers)
Open AccessReview
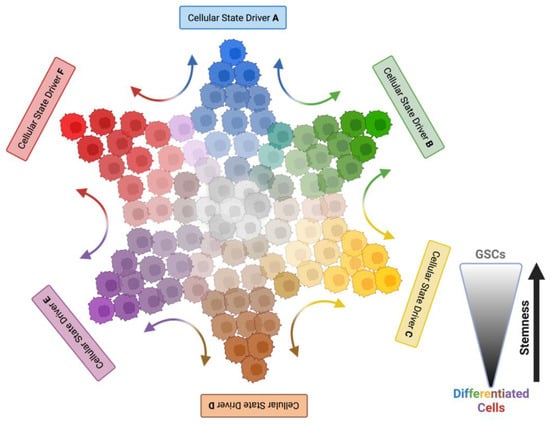
Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance
by
Frank Eckerdt and Leonidas C. Platanias
Cited by 5 | Viewed by 3337
Abstract
Since their discovery at the beginning of this millennium, glioma stem cells (GSCs) have sparked extensive research and an energetic scientific debate about their contribution to glioblastoma (GBM) initiation, progression, relapse, and resistance. Different molecular subtypes of GBM coexist within the same tumor,
[...] Read more.
Since their discovery at the beginning of this millennium, glioma stem cells (GSCs) have sparked extensive research and an energetic scientific debate about their contribution to glioblastoma (GBM) initiation, progression, relapse, and resistance. Different molecular subtypes of GBM coexist within the same tumor, and they display differential sensitivity to chemotherapy. GSCs contribute to tumor heterogeneity and recapitulate pathway alterations described for the three GBM subtypes found in patients. GSCs show a high degree of plasticity, allowing for interconversion between different molecular GBM subtypes, with distinct proliferative potential, and different degrees of self-renewal and differentiation. This high degree of plasticity permits adaptation to the environmental changes introduced by chemo- and radiation therapy. Evidence from mouse models indicates that GSCs repopulate brain tumors after therapeutic intervention, and due to GSC plasticity, they reconstitute heterogeneity in recurrent tumors. GSCs are also inherently resilient to standard-of-care therapy, and mechanisms of resistance include enhanced DNA damage repair, MGMT promoter demethylation, autophagy, impaired induction of apoptosis, metabolic adaptation, chemoresistance, and immune evasion. The remarkable oncogenic properties of GSCs have inspired considerable interest in better understanding GSC biology and functions, as they might represent attractive targets to advance the currently limited therapeutic options for GBM patients. This has raised expectations for the development of novel targeted therapeutic approaches, including targeting GSC plasticity, chimeric antigen receptor T (CAR T) cells, and oncolytic viruses. In this review, we focus on the role of GSCs as drivers of GBM and therapy resistance, and we discuss how insights into GSC biology and plasticity might advance GSC-directed curative approaches.
Full article
►▼
Show Figures
Open AccessArticle
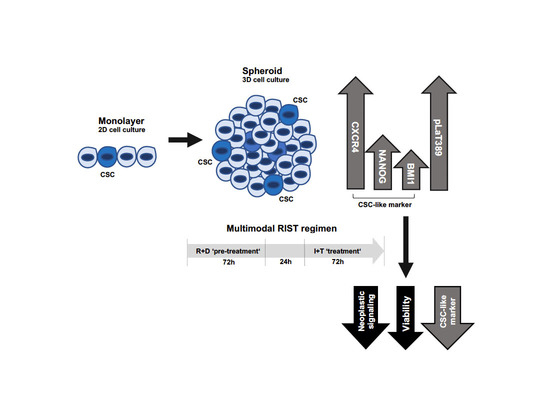
Evaluating the RIST Molecular-Targeted Regimen in a Three-Dimensional Neuroblastoma Spheroid Cell Culture Model
by
Carina Kaess, Marie Matthes, Jonas Gross, Rebecca Waetzig, Tilman Heise, Selim Corbacioglu and Gunhild Sommer
Cited by 1 | Viewed by 1775
Abstract
Background: The outcome for patients with high-risk neuroblastoma remains poor and novel treatment strategies are urgently needed. The RIST protocol represents a novel metronomic and multimodal treatment strategy for high-risk neuroblastoma combining molecular-targeted drugs as ‘pre-treatment’ with a conventional chemotherapy backbone, currently evaluated
[...] Read more.
Background: The outcome for patients with high-risk neuroblastoma remains poor and novel treatment strategies are urgently needed. The RIST protocol represents a novel metronomic and multimodal treatment strategy for high-risk neuroblastoma combining molecular-targeted drugs as ‘pre-treatment’ with a conventional chemotherapy backbone, currently evaluated in a phase II clinical trial. For preclinical drug testing, cancer cell growth as spheroid compared to mo-nolayer cultures is of advantage since it reproduces a wide range of tumor characteristics, including the three-dimensional architecture and cancer stem cell (CSC) properties. The objective of this study was to establish a neuroblastoma spheroid model for the rigorous assessment of the RIST treatment protocol. Methods: Evaluation of CSC marker expression was performed by mRNA and protein analysis and spheroid viability by luminescence-based assays. Aberrant expression of RNA-binding protein La in neuroblastoma was assessed by tissue microarray analysis and patients’ data mining. Results: Spheroid cultures showed increased expression of a subgroup of CSC-like markers (CXCR4, NANOG and BMI) and higher Thr389 phosphorylation of the neuroblastoma-associated RNA-binding protein La when compared to monolayer cultures. Molecular-targeted ‘pre-treatment’ of spheroids decreased neoplastic signaling and CSC marker expression. Conclusions: The RIST treatment protocol efficiently reduced the viability of neuroblastoma spheroids characterized by advanced CSC properties.
Full article
►▼
Show Figures
Open AccessArticle
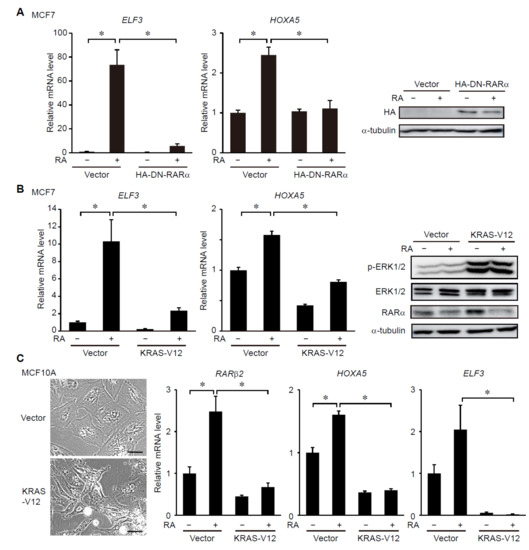
ERK MAP Kinase Signaling Regulates RAR Signaling to Confer Retinoid Resistance on Breast Cancer Cells
by
Akira Hirota, Jean-Emmanuel Clément, Satoshi Tanikawa, Takayuki Nonoyama, Tamiki Komatsuzaki, Jian Ping Gong, Shinya Tanaka and Masamichi Imajo
Viewed by 2239
Abstract
Retinoic acid (RA) and its synthetic derivatives, retinoids, have been established as promising anticancer agents based on their ability to regulate cell proliferation and survival. Clinical trials, however, have revealed that cancer cells often acquire resistance to retinoid therapy. Therefore, elucidation of underlying
[...] Read more.
Retinoic acid (RA) and its synthetic derivatives, retinoids, have been established as promising anticancer agents based on their ability to regulate cell proliferation and survival. Clinical trials, however, have revealed that cancer cells often acquire resistance to retinoid therapy. Therefore, elucidation of underlying mechanisms of retinoid resistance has been considered key to developing more effective use of retinoids in cancer treatment. In this study, we show that constitutive activation of ERK MAP kinase signaling, which is often caused by oncogenic mutations in
RAS or
RAF genes, suppresses RA receptor (RAR) signaling in breast cancer cells. We show that activation of the ERK pathway suppresses, whereas its inhibition promotes, RA-induced transcriptional activation of RAR and the resultant upregulation of RAR-target genes in breast cancer cells. Importantly, ERK inhibition potentiates the tumor-suppressive activity of RA in breast cancer cells. Moreover, we also reveal that suppression of RAR signaling and activation of ERK signaling are associated with poor prognoses in breast cancer patients and represent hallmarks of specific subtypes of breast cancers, such as basal-like, HER2-enriched and luminal B. These results indicate that ERK-dependent suppression of RAR activity underlies retinoid resistance and is associated with cancer subtypes and patient prognosis in breast cancers.
Full article
►▼
Show Figures
Open AccessArticle
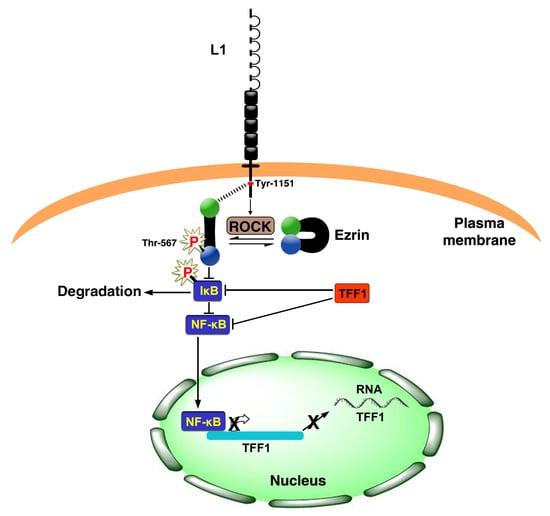
Downregulation of the Tumor Suppressor TFF1 Is Required during Induction of Colon Cancer Progression by L1
by
Arka Saha, Nancy Gavert, Thomas Brabletz and Avri Ben-Ze’ev
Viewed by 1965
Abstract
The immunoglobulin family cell adhesion receptor L1 is induced in CRC cells at the invasive front of the tumor tissue, and confers enhanced proliferation, motility, tumorigenesis, and liver metastasis. To identify putative tumor suppressors whose expression is downregulated in L1-expressing CRC cells, we
[...] Read more.
The immunoglobulin family cell adhesion receptor L1 is induced in CRC cells at the invasive front of the tumor tissue, and confers enhanced proliferation, motility, tumorigenesis, and liver metastasis. To identify putative tumor suppressors whose expression is downregulated in L1-expressing CRC cells, we blocked the L1–ezrin–NF-κB signaling pathway and searched for genes induced under these conditions. We found that TFF1, a protein involved in protecting the mucus epithelial layer of the colon, is downregulated in L1-expressing cells and displays characteristics of a tumor suppressor. Overexpression of TFF1 in L1-transfected human CRC cells blocks the pro-tumorigenic and metastatic properties conferred by L1 by suppressing NF-κB signaling. Immunohistochemical analyses revealed that human CRC tissue samples often lose the expression of TFF1, while the normal mucosa displays TFF1 in goblet cells. Identifying TFF1 as a tumor suppressor in CRC cells could provide a novel marker for L1-mediated CRC development and a potential target for therapy.
Full article
►▼
Show Figures
Open AccessReview
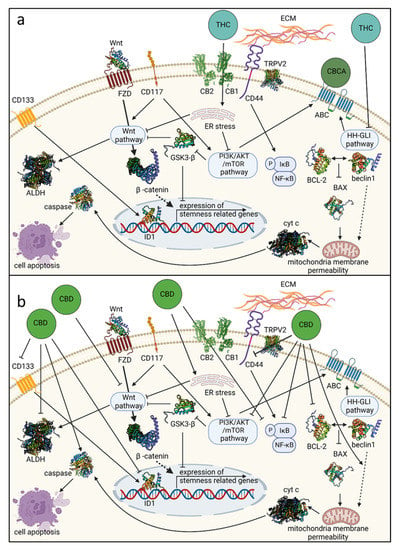
Anti-Cancer Activity of Cannabis sativa Phytocannabinoids: Molecular Mechanisms and Potential in the Fight against Ovarian Cancer and Stem Cells
by
Hinanit Koltai and Nurit Shalev
Cited by 8 | Viewed by 4066
Abstract
Ovarian cancer (OC) is the most lethal gynecological malignancy, with about 70% of cases diagnosed only at an advanced stage.
Cannabis sativa, which produces more than 150 phytocannabinoids, is used worldwide to alleviate numerous symptoms associated with various medical conditions. Recently, studies
[...] Read more.
Ovarian cancer (OC) is the most lethal gynecological malignancy, with about 70% of cases diagnosed only at an advanced stage.
Cannabis sativa, which produces more than 150 phytocannabinoids, is used worldwide to alleviate numerous symptoms associated with various medical conditions. Recently, studies across a range of cancer types have demonstrated that the phytocannabinoids Δ
9-trans-tetrahydrocannabinol (THC) and cannabidiol (CBD) have anti-cancer activity in vitro and in vivo, but also the potential to increase other drugs’ adverse effects. THC and CBD act via several different biological and signaling pathways, including receptor-dependent and receptor-independent pathways. However, very few studies have examined the effectiveness of cannabis compounds against OC. Moreover, little is known about the effectiveness of cannabis compounds against cancer stem cells (CSCs) in general and OC stem cells (OCSCs) in particular. CSCs have been implicated in tumor initiation, progression, and invasion, as well as tumor recurrence, metastasis, and drug resistance. Several hallmarks and concepts describe CSCs. OCSCs, too, are characterized by several markers and specific drug-resistance mechanisms. While there is no peer-reviewed information regarding the effect of cannabis and cannabis compounds on OCSC viability or development, cannabis compounds have been shown to affect genetic pathways and biological processes related to CSCs and OCSCs. Based on evidence from other cancer-type studies, the use of phytocannabinoid-based treatments to disrupt CSC homeostasis is suggested as a potential intervention to prevent chemotherapy resistance. The potential benefits of the combination of chemotherapy with phytocannabinoid treatment should be examined in ovarian cancer patients.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
Challenges for Triple Negative Breast Cancer Treatment: Defeating Heterogeneity and Cancer Stemness
by
Rinad Mahmoud, Paloma Ordóñez-Morán and Cinzia Allegrucci
Cited by 17 | Viewed by 8302
Abstract
The Triple Negative Breast Cancer (TNBC) subtype is known to have a more aggressive clinical course compared to other breast cancer subtypes. Targeted therapies for this type of breast cancer are limited and patients are mostly treated with conventional chemo- and radio-therapies which
[...] Read more.
The Triple Negative Breast Cancer (TNBC) subtype is known to have a more aggressive clinical course compared to other breast cancer subtypes. Targeted therapies for this type of breast cancer are limited and patients are mostly treated with conventional chemo- and radio-therapies which are not specific and do not target resistant cells. Therefore, one of the major clinical challenges is to find compounds that target the drug-resistant cell populations which are responsible for reforming secondary tumours. The molecular profiling of the different TNBC subtypes holds a promise for better defining these resistant cells specific to each tumour. To this end, a better understanding of TNBC heterogeneity and cancer stemness is required, and extensive genomic analysis can help to understand the disease complexity and distinguish new molecular drivers that can be targeted in the clinics. The use of persister cancer cell-targeting therapies combined with other therapies may provide a big advance to improve TNBC patients’ survival.
Full article
►▼
Show Figures
Open AccessReview
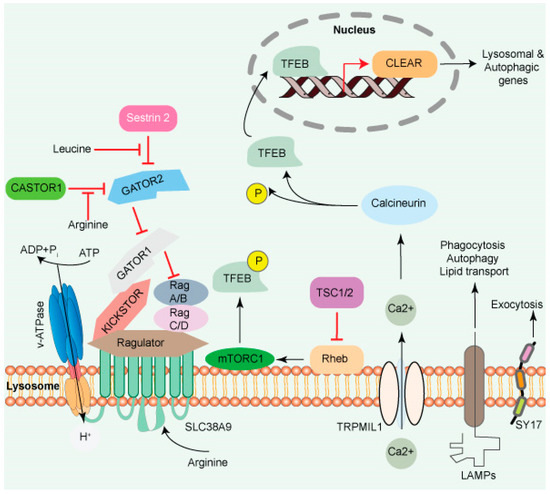
Lysosomes in Stem Cell Quiescence: A Potential Therapeutic Target in Acute Myeloid Leukemia
by
Vaibhav Jain, Swaroop Bose, Awadhesh K. Arya and Tasleem Arif
Cited by 5 | Viewed by 6696
Abstract
Lysosomes are cellular organelles that regulate essential biological processes such as cellular homeostasis, development, and aging. They are primarily connected to the degradation/recycling of cellular macromolecules and participate in cellular trafficking, nutritional signaling, energy metabolism, and immune regulation. Therefore, lysosomes connect cellular metabolism
[...] Read more.
Lysosomes are cellular organelles that regulate essential biological processes such as cellular homeostasis, development, and aging. They are primarily connected to the degradation/recycling of cellular macromolecules and participate in cellular trafficking, nutritional signaling, energy metabolism, and immune regulation. Therefore, lysosomes connect cellular metabolism and signaling pathways. Lysosome’s involvement in the critical biological processes has rekindled clinical interest towards this organelle for treating various diseases, including cancer. Recent research advancements have demonstrated that lysosomes also regulate the maintenance and hemostasis of hematopoietic stem cells (HSCs), which play a critical role in the progression of acute myeloid leukemia (AML) and other types of cancer. Lysosomes regulate both HSCs’ metabolic networks and identity transition. AML is a lethal type of blood cancer with a poor prognosis that is particularly associated with aging. Although the genetic landscape of AML has been extensively described, only a few targeted therapies have been produced, warranting the need for further research. This review summarizes the functions and importance of targeting lysosomes in AML, while highlighting the significance of lysosomes in HSCs maintenance.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Anti-cancer activity of phytocannabinoids: implications and possibilities in the fight against ovarian cancer and stem cells
Authors: Hinanit Koltai and Nurit Shalev
Affiliation: ARO, Volcani Institute
Abstract: Ovarian cancer (OC) is the most lethal gynecologic malignancy, with about 70% of cases diagnosed at an advanced stage. Cannabis sativa is being used worldwide to alleviate numerous symptoms associated with medical conditions. C. sativa produces around 600 different molecules, including more than 150 phytocannabinoids and hundreds of terpenes. Recently, a large number of studies have demonstrated that many phytocannabinoids have anticancer activity both in vitro and in vivo, with the active compounds and the pathways affected varying greatly among cancer types. However, few studies have examined the effectiveness of cannabis compounds against OC. We review preclinical evidence and the few clinical studies for cannabis anti-OC activity, emphasizing the need to identify the active cannabis compounds, to examine co-treatment of cannabis and targeted-therapy drugs and to provide an insight into the mode of action. The potential for phytocannabinoids to particularly target stemness pathways in OC stem cells (OCSC) will be discussed. The effectiveness of cannabis compounds in combination with anti-OC drugs should be examined in clinical trials.
Title: xxxx
Authors: Natalia Koralewska Lucyna Budźko, Ireneusz Stolarek, Marek Figlerowicz
Affiliation: Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, 61-704, Poznan, Poland
Abstract: Stemness refers to the molecular processes underlying the core stem cell properties of self-renewal and generation of differentiated descendants. During the last decades cancer stem cells i.e. cancer cells showing stemness traits were identified in most types of solid and hematologic cancers.
Compelling evidence has demonstrated that cancer stem cells behave as malignant equivalents of adult stem cells and act as a driving force of tumorigenesis. For example, hematological cancers, such as leukemias, are derived from hematopoietic stem and progenitor cells by the acquisition of genetic and epigenetic aberrations that reprogram and subsequently transform the hematopoietic system.
For many years the research was focused on the genomic background of cancer which limited the understanding of the cancer stemness phenomenon. It was partially due to the methodological incapabilities. Recently, we have witnessed a rapid development of multiomics and single-cell techniques providing multiple modalities that open a new era in studying cancer stem cells.
Accordingly, in this review, we focus on recent advances in cancer stem cell studies highlighting the interplay between epigenomics, genomics, and cell-to-cell signaling. We will put a special emphasis on mechanisms relevant to stem cell formation and function, especially in hematological cancers. We discuss the current line of work on cancer stemness from a cell-based view, focusing on cancer stem cells fate and their involvement in disease progression. We explore differences between normal and malignant stem cells and give a more focused view on the therapy-induced cancer stemness involving cellular senescence. We postulate that cancer stem cell plasticity opens immense possibilities for earlier detection and more effective treatment of cancer through cell-based interceptive medicine.