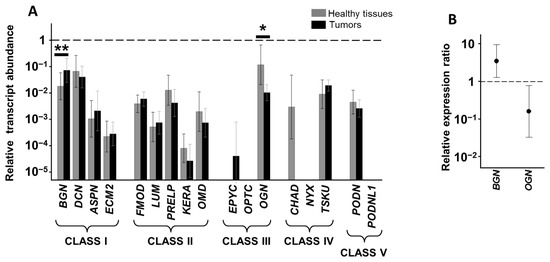
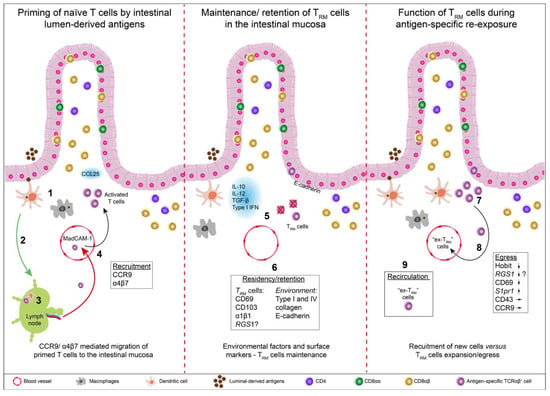
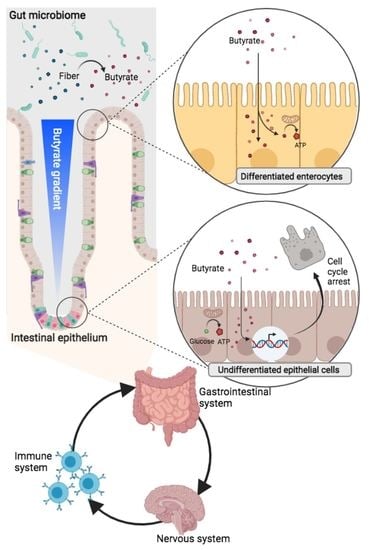
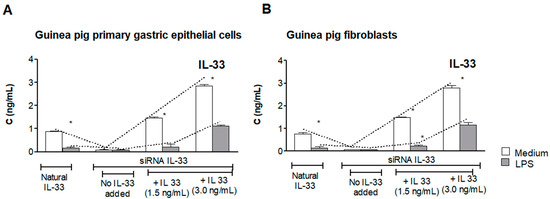
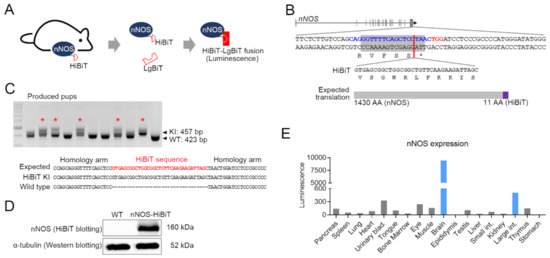
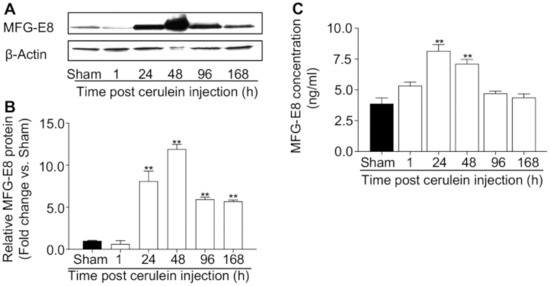
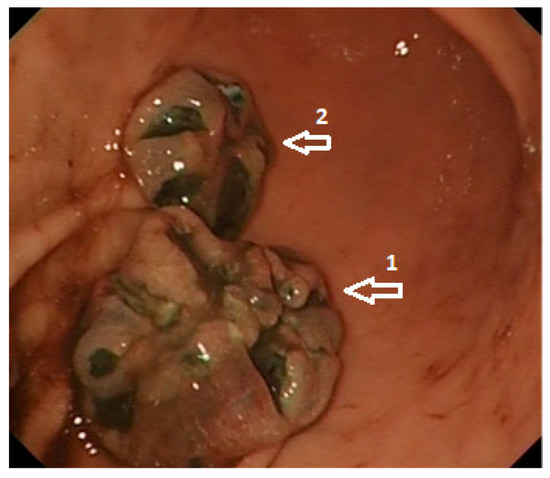
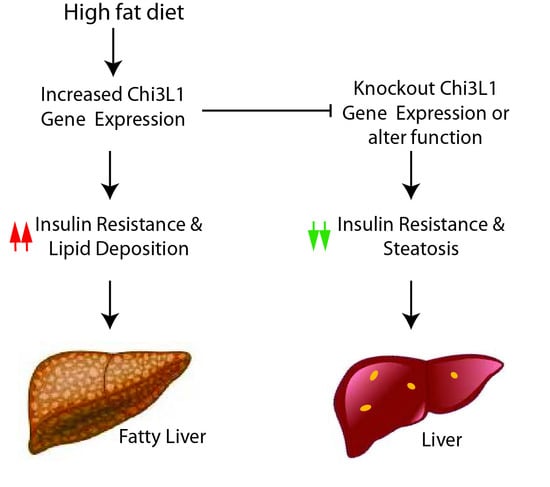
Gastrointestinal Disease: From Molecular and Cellular Mechanisms to Novel Therapeutics
A topical collection in Cells (ISSN 2073-4409).
Viewed by 109820Editor
Topical Collection Information
Dear Colleagues,
Recent advances in cell and molecular biology have dramatically illuminated the pathophysiology of the GI tract across a wide range of diseases from injury and repair to reperfusion injury to chronic inflammatory states such as inflammatory bowel disease or pancreatitis or hepatitis to cancer. New technology and new work is helping us to identify new mechanisms of such pathophysiology and, thus, potential new targets for therapeutic intervention. In this context, it seems timely to assemble a compendium of some recent advances in our understanding of the pathobiology of GI disease. We invite you to contribute your work or nominate others who you think ought to be invited to contribute to a Topical Collection of Cells on “Gastrointestinal Disease: From Molecular and Cellular Mechanisms to Novel Therapeutics”. This peer-reviewed Topical Collection will showcase exciting new work in the field that may foreshadow the therapeutics of the future. We hope that you will consider contributing.
Prof. Marc BassonCollection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Stomach
- Intestine
- Liver
- Pancreas
- Mucosa
- Cancer
- Mucosal repair
- Wound healing