STIM and Orai Communication in Health and Disease
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 53031
Share This Topical Collection
Editor
 Dr. Isabella Derler
Dr. Isabella Derler
 Dr. Isabella Derler
Dr. Isabella Derler
E-Mail
Website
Collection Editor
Institute of Biophysics, Johannes Kepler University Linz, Linz, Austria
Interests: Ca2+ signalling; Ca2+ ion channels: Orai and STIM proteins, TRP proteins; voltage-gated Ca2+ ion channels; Ca2+-activated potassium ion channels; ion channel structure–function relationships; optogenetics
Topical Collection Information
Dear Colleagues,
Accurately tuned intracellular Ca2+ signaling is indispensable for the regulation of a plethora of cellular processes occurring over a wide temporal range, including for instance neuronal signaling, proliferation, and gene transcription. The multiple Ca2+ signaling pathways are established via a huge toolkit of Ca2+-signaling proteins. A single defect of one of these molecular key players can lead to abnormal cytosolic Ca2+ levels, which can be responsible for severe disorders in the immune system, heart function, and neurons or even for cancer. A prominent Ca2+ signaling pathway in the cell represents the CRAC channel, composed of two molecular key STIM1 players, a Ca2+ sensor in the endoplasmic reticulum, and Orai1, a highly Ca2+ selective ion channel in the plasma membrane. These two proteins and their isoforms have been known for more than a decade, and a series of milestones have been reached in the understanding of their structure–function relationship, interplay, and co-regulation with modulatory factors as well as their significance in disease.
Herewith, I would like to cordially invite you all to contribute to this Topic Collection on “STIM1 and Orai1 Communication in Health and Disease” in the form of experimental papers on your latest research, up-to-date research articles, and commentaries. This issue of Cells will specifically focus on novel findings in the field of store-operated Ca2+ ion channels in both health and disease.
Dr. Isabella Derler
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Store-operated Ca2+ ion channels
- CRAC
- STIM1, STIM2
- Orai1, Orai2, Orai3
- Diseases associated with defect Ca2+ signaling
Published Papers (18 papers)
Open AccessReview
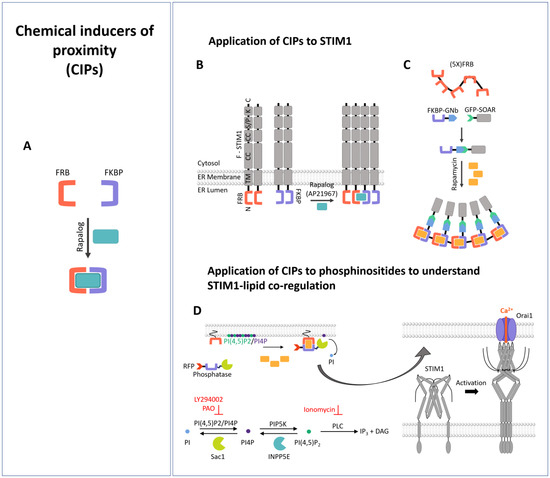
Synthetic Biology Meets Ca2+ Release-Activated Ca2+ Channel-Dependent Immunomodulation
by
Bernadett Bacsa, Valentina Hopl and Isabella Derler
Viewed by 940
Abstract
Many essential biological processes are triggered by the proximity of molecules. Meanwhile, diverse approaches in synthetic biology, such as new biological parts or engineered cells, have opened up avenues to precisely control the proximity of molecules and eventually downstream signaling processes. This also
[...] Read more.
Many essential biological processes are triggered by the proximity of molecules. Meanwhile, diverse approaches in synthetic biology, such as new biological parts or engineered cells, have opened up avenues to precisely control the proximity of molecules and eventually downstream signaling processes. This also applies to a main Ca
2+ entry pathway into the cell, the so-called Ca
2+ release-activated Ca
2+ (CRAC) channel. CRAC channels are among other channels are essential in the immune response and are activated by receptor–ligand binding at the cell membrane. The latter initiates a signaling cascade within the cell, which finally triggers the coupling of the two key molecular components of the CRAC channel, namely the stromal interaction molecule, STIM, in the ER membrane and the plasma membrane Ca
2+ ion channel, Orai. Ca
2+ entry, established via STIM/Orai coupling, is essential for various immune cell functions, including cytokine release, proliferation, and cytotoxicity. In this review, we summarize the tools of synthetic biology that have been used so far to achieve precise control over the CRAC channel pathway and thus over downstream signaling events related to the immune response.
Full article
►▼
Show Figures
Open AccessArticle
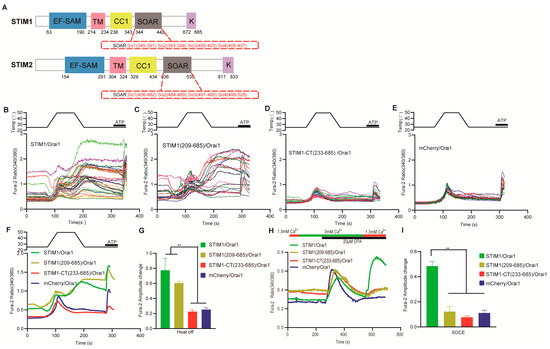
Molecular Mechanism Analysis of STIM1 Thermal Sensation
by
Xiaoling Liu, Tianyuan Zheng, Yan Jiang, Lei Wang, Yuchen Zhang, Qiyu Liang and Yuejie Chen
Viewed by 811
Abstract
STIM1 has been identified as a new warm sensor, but the exact molecular mechanism remains unclear. In this study, a variety of mutants of STIM1, Orai1 and Orai3 were generated. The single–cell calcium imaging and confocal analysis were used to evaluate the thermal
[...] Read more.
STIM1 has been identified as a new warm sensor, but the exact molecular mechanism remains unclear. In this study, a variety of mutants of STIM1, Orai1 and Orai3 were generated. The single–cell calcium imaging and confocal analysis were used to evaluate the thermal sensitivity of the resulting STIM mutants and the interaction between STIM1 and Orai mutants in response to temperature. Our results suggested that the CC1–SOAR of STIM1 was a direct activation domain of temperature, leading to subsequent STIM1 activation, and the transmembrane (TM) region and K domain but not EF–SAM were needed for this process. Furthermore, both the TM and SOAR domains exhibited similarities and differences between STIM1–mediated thermal sensation and store–operated calcium entry (SOCE), and the key sites of Orai1 showed similar roles in these two responses. Additionally, the TM23 (comprising TM2, loop2, and TM3) region of Orai1 was identified as the key domain determining the STIM1/Orai1 thermal response pattern, while the temperature reactive mode of STIM1/Orai3 seemed to result from a combined effect of Orai3. These findings provide important support for the specific molecular mechanism of STIM1–induced thermal response, as well as the interaction mechanism of STIM1 with Orai1 and Orai3 after being activated by temperature.
Full article
►▼
Show Figures
Open AccessArticle
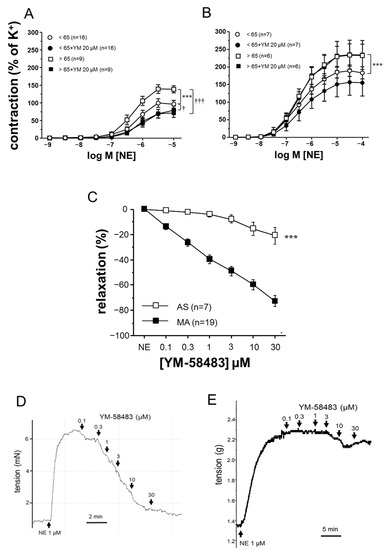
Functional Role of STIM-1 and Orai1 in Human Microvascular Aging
by
Mariam El Assar, Esther García-Rojo, Alejandro Sevilleja-Ortiz, Alberto Sánchez-Ferrer, Argentina Fernández, Borja García-Gómez, Javier Romero-Otero, Leocadio Rodríguez-Mañas and Javier Angulo
Cited by 3 | Viewed by 1174
Abstract
The impact of aging on vascular function is heterogeneous depending on the vascular territories. Calcium regulation plays a key role in vascular function and has been implicated in aging-related hypercontractility of corpus cavernosum. We aimed to evaluate stromal interaction molecule (STIM)/Orai system involvement
[...] Read more.
The impact of aging on vascular function is heterogeneous depending on the vascular territories. Calcium regulation plays a key role in vascular function and has been implicated in aging-related hypercontractility of corpus cavernosum. We aimed to evaluate stromal interaction molecule (STIM)/Orai system involvement in aging-related vascular alterations in the human macro and microvasculature. Aortae specimens and mesenteric arteries (MA), obtained from 45 organ donors, were functionally evaluated in organ chambers and wire myographs. Subjects were divided into groups either younger or older than 65-years old. The expressions of STIM-1, Orai1, and Orai3 were determined by immunofluorescence in the aorta and MA, and by Western blot in the aorta homogenates. The inhibition of STIM/Orai with YM-58483 (20 μM) reversed adrenergic hypercontractility in MA from older subjects but did not modify aging-related hypercontractility in the aortic strips. Aging was related to an increased expression of Orai1 in human aorta, while Orai1 and STIM-1 were upregulated in MA. STIM-1 and Orai1 protein expressions were inversely correlated to endothelial function in MA. Circulating levels of Orai1 were correlated with the inflammatory factor TNF-α and with the endothelial dysfunction marker asymmetric dimethylarginine. Aging is associated with an increased expression of the STIM/Orai system in human vessels with functional relevance only in the microvascular territory, suggesting its role in aging-related microvascular dysfunction.
Full article
►▼
Show Figures
Open AccessReview
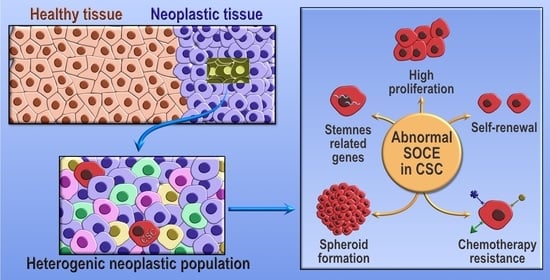
Store-Operated Calcium Entry and Its Implications in Cancer Stem Cells
by
Isaac Jardin, Jose J. Lopez, Jose Sanchez-Collado, Luis J. Gomez, Gines M. Salido and Juan A. Rosado
Cited by 8 | Viewed by 2659
Abstract
Tumors are composed by a heterogeneous population of cells. Among them, a sub-population of cells, termed cancer stem cells, exhibit stemness features, such as self-renewal capabilities, disposition to differentiate to a more proliferative state, and chemotherapy resistance, processes that are all mediated by
[...] Read more.
Tumors are composed by a heterogeneous population of cells. Among them, a sub-population of cells, termed cancer stem cells, exhibit stemness features, such as self-renewal capabilities, disposition to differentiate to a more proliferative state, and chemotherapy resistance, processes that are all mediated by Ca
2+. Ca
2+ homeostasis is vital for several physiological processes, and alterations in the patterns of expressions of the proteins and molecules that modulate it have recently become a cancer hallmark. Store-operated Ca
2+ entry is a major mechanism for Ca
2+ entry from the extracellular medium in non-excitable cells that leads to increases in the cytosolic Ca
2+ concentration required for several processes, including cancer stem cell properties. Here, we focus on the participation of STIM, Orai, and TRPC proteins, the store-operated Ca
2+ entry key components, in cancer stem cell biology and tumorigenesis.
Full article
►▼
Show Figures
Open AccessReview
On the Connections between TRPM Channels and SOCE
by
Guilherme H. Souza Bomfim, Barbara A. Niemeyer, Rodrigo S. Lacruz and Annette Lis
Cited by 3 | Viewed by 2839
Abstract
Plasma membrane protein channels provide a passageway for ions to access the intracellular milieu. Rapid entry of calcium ions into cells is controlled mostly by ion channels, while Ca
2+-ATPases and Ca
2+ exchangers ensure that cytosolic Ca
2+ levels ([Ca
2+
[...] Read more.
Plasma membrane protein channels provide a passageway for ions to access the intracellular milieu. Rapid entry of calcium ions into cells is controlled mostly by ion channels, while Ca
2+-ATPases and Ca
2+ exchangers ensure that cytosolic Ca
2+ levels ([Ca
2+]
cyt) are maintained at low (~100 nM) concentrations. Some channels, such as the Ca
2+-release-activated Ca
2+ (CRAC) channels and voltage-dependent Ca
2+ channels (CACNAs), are highly Ca
2+-selective, while others, including the Transient Receptor Potential Melastatin (TRPM) family, have broader selectivity and are mostly permeable to monovalent and divalent cations. Activation of CRAC channels involves the coupling between ORAI1-3 channels with the endoplasmic reticulum (ER) located Ca
2+ store sensor, Stromal Interaction Molecules 1-2 (STIM1/2), a pathway also termed store-operated Ca
2+ entry (SOCE). The TRPM family is formed by 8 members (TRPM1-8) permeable to Mg
2+, Ca
2+, Zn
2+ and Na
+ cations, and is activated by multiple stimuli. Recent studies indicated that SOCE and TRPM structure-function are interlinked in some instances, although the molecular details of this interaction are only emerging. Here we review the role of TRPM and SOCE in Ca
2+ handling and highlight the available evidence for this interaction.
Full article
►▼
Show Figures
Open AccessReview
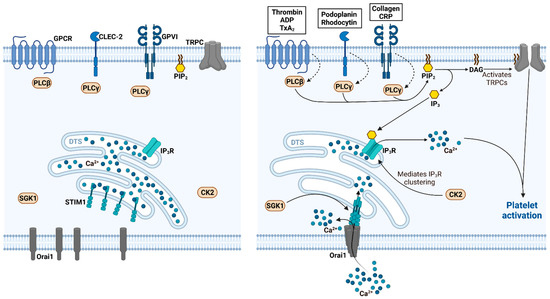
CRACking the Molecular Regulatory Mechanism of SOCE during Platelet Activation in Thrombo-Occlusive Diseases
by
Patrick Münzer and Oliver Borst
Cited by 2 | Viewed by 2863
Abstract
Thrombo-occlusive diseases such as myocardial infarction, ischemic stroke and deep vein thrombosis with subsequent pulmonary embolism still represent a major health burden worldwide. Besides the cells of the vasculature or other hematopoietic cells, platelets are primarily responsible for the development and progression of
[...] Read more.
Thrombo-occlusive diseases such as myocardial infarction, ischemic stroke and deep vein thrombosis with subsequent pulmonary embolism still represent a major health burden worldwide. Besides the cells of the vasculature or other hematopoietic cells, platelets are primarily responsible for the development and progression of an occluding thrombus. The activation and function of platelets crucially depend on free cytosolic calcium (Ca
2+) as second messenger, which modulates platelet secretion, aggregation and thrombus formation. Ca
2+ is elevated upon platelet activation by release of Ca
2+ from intracellular stores thus triggering of the subsequent store-operated Ca
2+ entry (SOCE), which is facilitated by Ca
2+ release-activated channels (CRACs). In general, CRACs are assembled by the pore-forming unit Orai in the plasma membrane and the Ca
2+-sensing stromal interaction molecule (STIM) in the endoplasmic reticulum after the depletion of internal Ca
2+ stores. In the last few years, there is a growing body of the literature demonstrating the importance of STIM and Orai-mediated mechanism in thrombo-occlusive disorders. Thus, this review provides an overview of the recent understanding of STIM and Orai signaling in platelet function and its implication in the development and progression of ischemic thrombo-occlusive disorders. Moreover, potential pharmacological implications of STIM and Orai signaling in platelets are anticipated and discussed in the end.
Full article
►▼
Show Figures
Open AccessReview
Highlighting the Multifaceted Role of Orai1 N-Terminal- and Loop Regions for Proper CRAC Channel Functions
by
Christina Humer, Christoph Romanin and Carmen Höglinger
Cited by 3 | Viewed by 2874
Abstract
Orai1, the Ca
2+-selective pore in the plasma membrane, is one of the key components of the Ca
2+release-activated Ca
2+ (CRAC) channel complex. Activated by the Ca
2+ sensor in the endoplasmic reticulum (ER) membrane, stromal interaction molecule 1 (STIM1),
[...] Read more.
Orai1, the Ca
2+-selective pore in the plasma membrane, is one of the key components of the Ca
2+release-activated Ca
2+ (CRAC) channel complex. Activated by the Ca
2+ sensor in the endoplasmic reticulum (ER) membrane, stromal interaction molecule 1 (STIM1), via direct interaction when ER luminal Ca
2+ levels recede, Orai1 helps to maintain Ca
2+ homeostasis within a cell. It has already been proven that the C-terminus of Orai1 is indispensable for channel activation. However, there is strong evidence that for CRAC channels to function properly and maintain all typical hallmarks, such as selectivity and reversal potential, additional parts of Orai1 are needed. In this review, we focus on these sites apart from the C-terminus; namely, the second loop and N-terminus of Orai1 and on their multifaceted role in the functioning of CRAC channels.
Full article
►▼
Show Figures
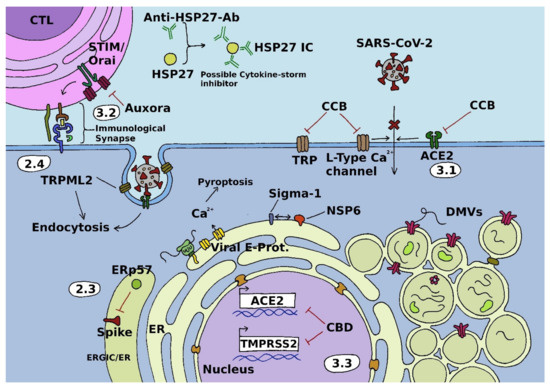
Open AccessFeature PaperReview
Calcium Signals during SARS-CoV-2 Infection: Assessing the Potential of Emerging Therapies
by
Sascha Berlansky, Matthias Sallinger, Herwig Grabmayr, Christina Humer, Andreas Bernhard, Marc Fahrner and Irene Frischauf
Cited by 23 | Viewed by 5565
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a positive-sense single-stranded RNA virus that causes coronavirus disease 2019 (COVID-19). This respiratory illness was declared a pandemic by the world health organization (WHO) in March 2020, just a few weeks after being described for
[...] Read more.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a positive-sense single-stranded RNA virus that causes coronavirus disease 2019 (COVID-19). This respiratory illness was declared a pandemic by the world health organization (WHO) in March 2020, just a few weeks after being described for the first time. Since then, global research effort has considerably increased humanity’s knowledge about both viruses and disease. It has also spawned several vaccines that have proven to be key tools in attenuating the spread of the pandemic and severity of COVID-19. However, with vaccine-related skepticism being on the rise, as well as breakthrough infections in the vaccinated population and the threat of a complete immune escape variant, alternative strategies in the fight against SARS-CoV-2 are urgently required. Calcium signals have long been known to play an essential role in infection with diverse viruses and thus constitute a promising avenue for further research on therapeutic strategies. In this review, we introduce the pivotal role of calcium signaling in viral infection cascades. Based on this, we discuss prospective calcium-related treatment targets and strategies for the cure of COVID-19 that exploit viral dependence on calcium signals.
Full article
►▼
Show Figures
Open AccessArticle
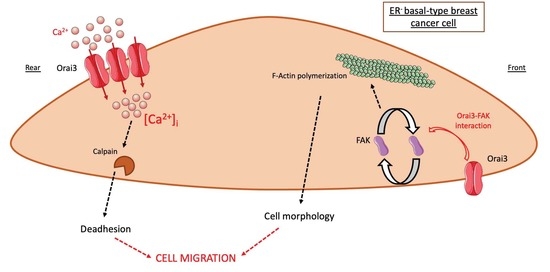
Orai3 Calcium Channel Regulates Breast Cancer Cell Migration through Calcium-Dependent and -Independent Mechanisms
by
Mohamed Chamlali, Sana Kouba, Lise Rodat-Despoix, Luca Matteo Todesca, Zoltán Pethö, Albrecht Schwab and Halima Ouadid-Ahidouch
Cited by 14 | Viewed by 3014
Abstract
Orai3 calcium (Ca
2+) channels are implicated in multiple breast cancer processes, such as proliferation and survival as well as resistance to chemotherapy. However, their involvement in the breast cancer cell migration processes remains vague. In the present study, we exploited MDA-MB-231
[...] Read more.
Orai3 calcium (Ca
2+) channels are implicated in multiple breast cancer processes, such as proliferation and survival as well as resistance to chemotherapy. However, their involvement in the breast cancer cell migration processes remains vague. In the present study, we exploited MDA-MB-231 and MDA-MB-231 BrM2 basal-like estrogen receptor-negative (ER
−) cell lines to assess the direct role of Orai3 in cell migration. We showed that Orai3 regulates MDA-MB-231 and MDA-MB-231 BrM2 cell migration in two distinct ways. First, we showed that Orai3 remodels cell adhesive capacities by modulating the intracellular Ca
2+ concentration. Orai3 silencing (siOrai3) decreased calpain activity, cell adhesion and migration in a Ca
2+-dependent manner. In addition, Orai3 interacts with focal adhesion kinase (FAK) and regulates the actin cytoskeleton, in a Ca
2+-independent way. Thus, siOrai3 modulates cell morphology by altering F-actin polymerization via a loss of interaction between Orai3 and FAK. To summarize, we demonstrated that Orai3 regulates cell migration through a Ca
2+-dependent modulation of calpain activity and, in a Ca
2+-independent manner, the actin cytoskeleton architecture via FAK.
Full article
►▼
Show Figures
Open AccessReview
Deciphering Molecular Mechanisms and Intervening in Physiological and Pathophysiological Processes of Ca2+ Signaling Mechanisms Using Optogenetic Tools
by
Lena Maltan, Hadil Najjar, Adéla Tiffner and Isabella Derler
Cited by 3 | Viewed by 2368
Abstract
Calcium ion channels are involved in numerous biological functions such as lymphocyte activation, muscle contraction, neurotransmission, excitation, hormone secretion, gene expression, cell migration, memory, and aging. Therefore, their dysfunction can lead to a wide range of cellular abnormalities and, subsequently, to diseases. To
[...] Read more.
Calcium ion channels are involved in numerous biological functions such as lymphocyte activation, muscle contraction, neurotransmission, excitation, hormone secretion, gene expression, cell migration, memory, and aging. Therefore, their dysfunction can lead to a wide range of cellular abnormalities and, subsequently, to diseases. To date various conventional techniques have provided valuable insights into the roles of Ca
2+ signaling. However, their limited spatiotemporal resolution and lack of reversibility pose significant obstacles in the detailed understanding of the structure–function relationship of ion channels. These drawbacks could be partially overcome by the use of optogenetics, which allows for the remote and well-defined manipulation of Ca
2+-signaling. Here, we review the various optogenetic tools that have been used to achieve precise control over different Ca
2+-permeable ion channels and receptors and associated downstream signaling cascades. We highlight the achievements of optogenetics as well as the still-open questions regarding the resolution of ion channel working mechanisms. In addition, we summarize the successes of optogenetics in manipulating many Ca
2+-dependent biological processes both in vitro and in vivo. In summary, optogenetics has significantly advanced our understanding of Ca
2+ signaling proteins and the used tools provide an essential basis for potential future therapeutic application.
Full article
►▼
Show Figures
Open AccessReview
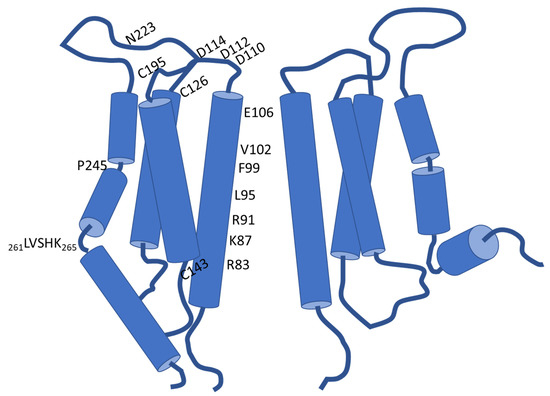
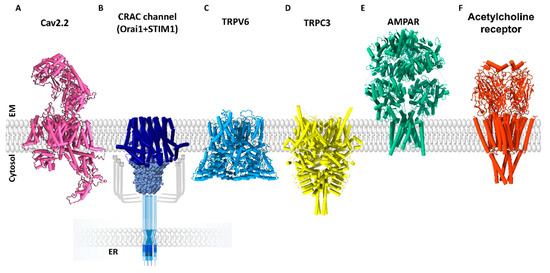
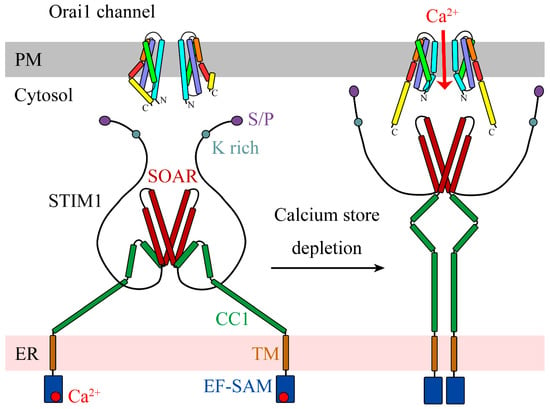
Structural Insights into Ca2+ Permeation through Orai Channels
by
Yang Li, Xue Yang and Yuequan Shen
Cited by 5 | Viewed by 1915
Abstract
Orai channels belong to the calcium release-activated calcium (CRAC) channel family. Orai channels are responsible for the influx of extracellular Ca
2+ that is triggered by Ca
2+ depletion from the endoplasmic reticulum (ER); this function is essential for many types of non-excitable
[...] Read more.
Orai channels belong to the calcium release-activated calcium (CRAC) channel family. Orai channels are responsible for the influx of extracellular Ca
2+ that is triggered by Ca
2+ depletion from the endoplasmic reticulum (ER); this function is essential for many types of non-excitable cells. Extensive structural and functional studies have advanced the knowledge of the molecular mechanism by which Orai channels are activated. However, the gating mechanism that allows Ca
2+ permeation through Orai channels is less well explained. Here, we reviewed and summarized the existing structural studies of Orai channels. We detailed the structural features of Orai channels, described structural comparisons of their closed and open states, and finally proposed a “push–pull” model of Ca
2+ permeation.
Full article
►▼
Show Figures
Open AccessArticle
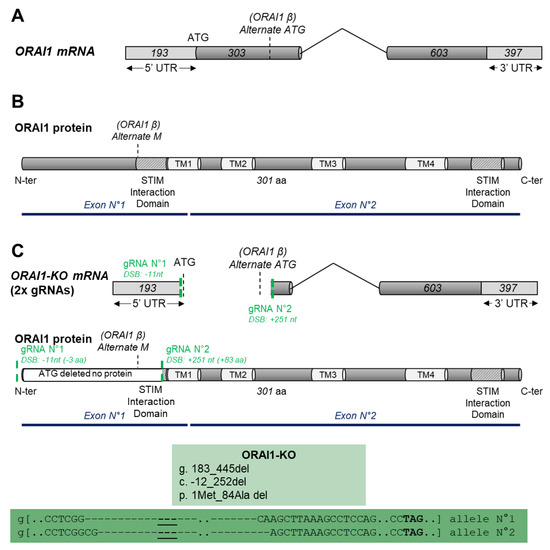
Impact of SOCE Abolition by ORAI1 Knockout on the Proliferation, Adhesion, and Migration of HEK-293 Cells
by
Alexandre Bokhobza, Nathalie Ziental-Gelus, Laurent Allart, Oksana Iamshanova and Fabien Vanden Abeele
Cited by 2 | Viewed by 2029
Abstract
Store-operated calcium entry (SOCE) provided through channels formed by ORAI proteins is a major regulator of several cellular processes. In immune cells, it controls fundamental processes such as proliferation, cell adhesion, and migration, while in cancer, SOCE and
ORAI1 gene expression are dysregulated
[...] Read more.
Store-operated calcium entry (SOCE) provided through channels formed by ORAI proteins is a major regulator of several cellular processes. In immune cells, it controls fundamental processes such as proliferation, cell adhesion, and migration, while in cancer, SOCE and
ORAI1 gene expression are dysregulated and lead to abnormal migration and/or cell proliferation. In the present study, we used the CRISPR/Cas9 technique to delete the
ORAI1 gene and to identify its role in proliferative and migrative properties of the model cell line HEK-293. We showed that ORAI1 deletion greatly reduced SOCE. Thereby, we found that this decrease and the absence of ORAI1 protein did not affect HEK-293 proliferation. In addition, we determined that ORAI1 suppression did not affect adhesive properties but had a limited impact on HEK-293 migration. Overall, we showed that ORAI1 and SOCE are largely dispensable for cellular proliferation, migration, and cellular adhesion of HEK-293 cells. Thus, despite its importance in providing Ca
2+ entry in non-excitable cells, our results indicate that the lack of SOCE does not deeply impact HEK-293 cells. This finding suggests the existence of compensatory mechanism enabling the maintenance of their physiological function.
Full article
►▼
Show Figures
Open AccessArticle
Calsequestrin 1 Is an Active Partner of Stromal Interaction Molecule 2 in Skeletal Muscle
by
Seung Yeon Jeong, Mi Ri Oh, Jun Hee Choi, Jin Seok Woo and Eun Hui Lee
Cited by 5 | Viewed by 2172
Abstract
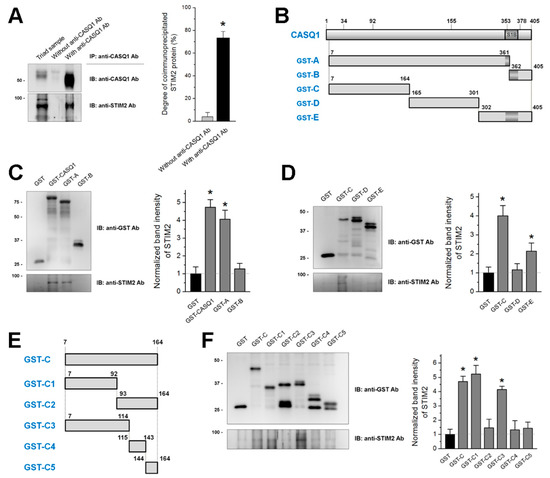
Calsequestrin 1 (CASQ1) in skeletal muscle buffers and senses Ca
2+ in the sarcoplasmic reticulum (SR). CASQ1 also regulates store-operated Ca
2+ entry (SOCE) by binding to stromal interaction molecule 1 (STIM1). Abnormal SOCE and/or abnormal expression or mutations in CASQ1, STIM1, or
[...] Read more.
Calsequestrin 1 (CASQ1) in skeletal muscle buffers and senses Ca
2+ in the sarcoplasmic reticulum (SR). CASQ1 also regulates store-operated Ca
2+ entry (SOCE) by binding to stromal interaction molecule 1 (STIM1). Abnormal SOCE and/or abnormal expression or mutations in CASQ1, STIM1, or STIM2 are associated with human skeletal, cardiac, or smooth muscle diseases. However, the functional relevance of CASQ1 along with STIM2 has not been studied in any tissue, including skeletal muscle. First, in the present study, it was found by biochemical approaches that CASQ1 is bound to STIM2 via its 92 N-terminal amino acids (C1 region). Next, to examine the functional relevance of the CASQ1-STIM2 interaction in skeletal muscle, the full-length wild-type CASQ1 or the C1 region was expressed in mouse primary skeletal myotubes, and the myotubes were examined using single-myotube Ca
2+ imaging experiments and transmission electron microscopy observations. The CASQ1-STIM2 interaction via the C1 region decreased SOCE, increased intracellular Ca
2+ release for skeletal muscle contraction, and changed intracellular Ca
2+ distributions (high Ca
2+ in the SR and low Ca
2+ in the cytosol were observed). Furthermore, the C1 region itself (which lacks Ca
2+-buffering ability but has STIM2-binding ability) decreased the expression of Ca
2+-related proteins (canonical-type transient receptor potential cation channel type 6 and calmodulin 1) and induced mitochondrial shape abnormalities. Therefore, in skeletal muscle, CASQ1 plays active roles in Ca
2+ movement and distribution by interacting with STIM2 as well as Ca
2+ sensing and buffering.
Full article
►▼
Show Figures
Open AccessReview
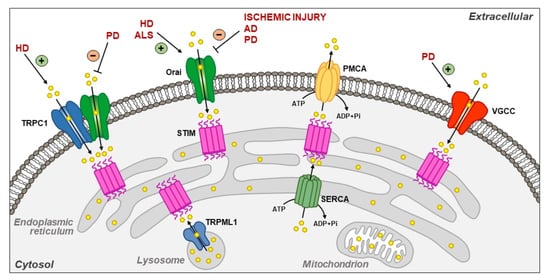
Plasma Membrane and Organellar Targets of STIM1 for Intracellular Calcium Handling in Health and Neurodegenerative Diseases
by
Valentina Tedeschi, Daniele La Russa, Cristina Franco, Antonio Vinciguerra, Diana Amantea and Agnese Secondo
Cited by 7 | Viewed by 2800
Abstract
Located at the level of the endoplasmic reticulum (ER) membrane, stromal interacting molecule 1 (STIM1) undergoes a complex conformational rearrangement after depletion of ER luminal Ca
2+. Then, STIM1 translocates into discrete ER-plasma membrane (PM) junctions where it directly interacts with and
[...] Read more.
Located at the level of the endoplasmic reticulum (ER) membrane, stromal interacting molecule 1 (STIM1) undergoes a complex conformational rearrangement after depletion of ER luminal Ca
2+. Then, STIM1 translocates into discrete ER-plasma membrane (PM) junctions where it directly interacts with and activates plasma membrane Orai1 channels to refill ER with Ca
2+. Furthermore, Ca
2+ entry due to Orai1/STIM1 interaction may induce canonical transient receptor potential channel 1 (TRPC1) translocation to the plasma membrane, where it is activated by STIM1. All these events give rise to store-operated calcium entry (SOCE). Besides the main pathway underlying SOCE, which mainly involves Orai1 and TRPC1 activation, STIM1 modulates many other plasma membrane proteins in order to potentiate the influxof Ca
2+. Furthermore, it is now clear that STIM1 may inhibit Ca
2+ currents mediated by L-type Ca
2+ channels. Interestingly, STIM1 also interacts with some intracellular channels and transporters, including nuclear and lysosomal ionic proteins, thus orchestrating organellar Ca
2+ homeostasis. STIM1 and its partners/effectors are significantly modulated in diverse acute and chronic neurodegenerative conditions. This highlights the importance of further disclosing their cellular functions as they might represent promising molecular targets for neuroprotection.
Full article
►▼
Show Figures
Open AccessReview
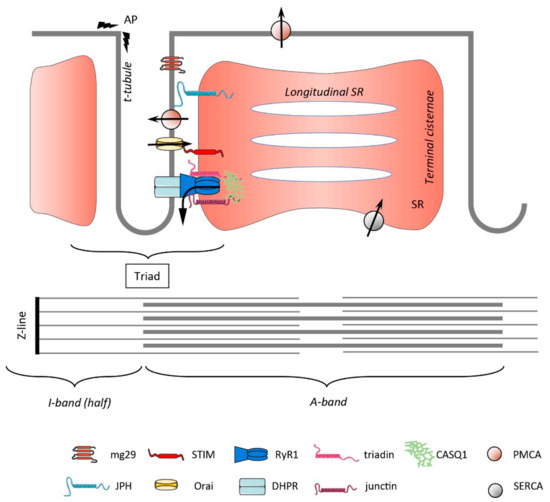
Store-Operated Calcium Entry in Skeletal Muscle: What Makes It Different?
by
Elena Lilliu, Stéphane Koenig, Xaver Koenig and Maud Frieden
Cited by 6 | Viewed by 4314
Abstract
Current knowledge on store-operated Ca
2+ entry (SOCE) regarding its localization, kinetics, and regulation is mostly derived from studies performed in non-excitable cells. After a long time of relative disinterest in skeletal muscle SOCE, this mechanism is now recognized as an essential contributor
[...] Read more.
Current knowledge on store-operated Ca
2+ entry (SOCE) regarding its localization, kinetics, and regulation is mostly derived from studies performed in non-excitable cells. After a long time of relative disinterest in skeletal muscle SOCE, this mechanism is now recognized as an essential contributor to muscle physiology, as highlighted by the muscle pathologies that are associated with mutations in the SOCE molecules STIM1 and Orai1. This review mainly focuses on the peculiar aspects of skeletal muscle SOCE that differentiate it from its counterpart found in non-excitable cells. This includes questions about SOCE localization and the movement of respective proteins in the highly organized skeletal muscle fibers, as well as the diversity of expressed STIM isoforms and their differential expression between muscle fiber types. The emerging evidence of a phasic SOCE, which is activated during EC coupling, and its physiological implication is described as well. The specific issues related to the use of SOCE modulators in skeletal muscles are discussed. This review highlights the complexity of SOCE activation and its regulation in skeletal muscle, with an emphasis on the most recent findings and the aim to reach a current picture of this mesmerizing phenomenon.
Full article
►▼
Show Figures
Open AccessReview
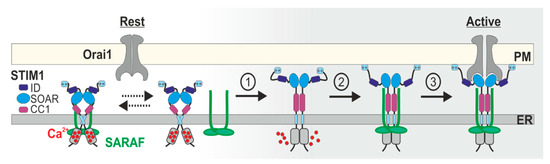
Regulation of Store-Operated Ca2+ Entry by SARAF
by
Inbal Dagan and Raz Palty
Cited by 6 | Viewed by 3362
Abstract
Calcium (Ca
2+) signaling plays a dichotomous role in cellular biology, controlling cell survival and proliferation on the one hand and cellular toxicity and cell death on the other. Store-operated Ca
2+ entry (SOCE) by CRAC channels represents a major pathway for
[...] Read more.
Calcium (Ca
2+) signaling plays a dichotomous role in cellular biology, controlling cell survival and proliferation on the one hand and cellular toxicity and cell death on the other. Store-operated Ca
2+ entry (SOCE) by CRAC channels represents a major pathway for Ca
2+ entry in non-excitable cells. The CRAC channel has two key components, the endoplasmic reticulum Ca
2+ sensor stromal interaction molecule (STIM) and the plasma-membrane Ca
2+ channel Orai. Physical coupling between STIM and Orai opens the CRAC channel and the resulting Ca
2+ flux is regulated by a negative feedback mechanism of slow Ca
2+ dependent inactivation (SCDI). The identification of the SOCE-associated regulatory factor (SARAF) and investigations of its role in SCDI have led to new functional and molecular insights into how SOCE is controlled. In this review, we provide an overview of the functional and molecular mechanisms underlying SCDI and discuss how the interaction between SARAF, STIM1, and Orai1 shapes Ca
2+ signaling in cells.
Full article
►▼
Show Figures
Open AccessArticle
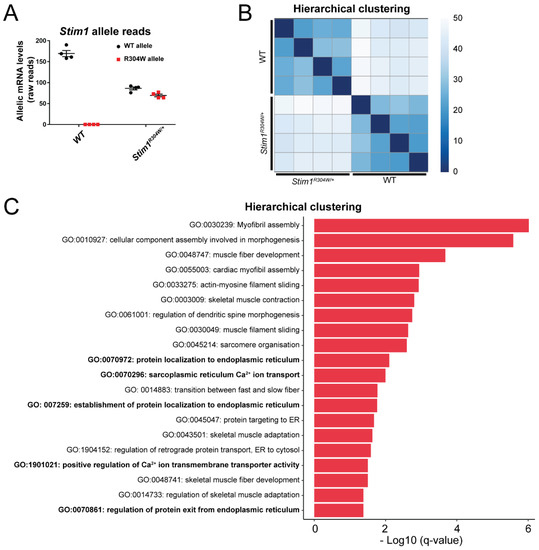
Pathophysiological Effects of Overactive STIM1 on Murine Muscle Function and Structure
by
Roberto Silva-Rojas, Anne-Laure Charles, Sarah Djeddi, Bernard Geny, Jocelyn Laporte and Johann Böhm
Cited by 11 | Viewed by 2931
Abstract
Store-operated Ca
2+ entry (SOCE) is a ubiquitous mechanism regulating extracellular Ca
2+ entry to control a multitude of Ca
2+-dependent signaling pathways and cellular processes. SOCE relies on the concerted activity of the reticular Ca
2+ sensor STIM1 and the plasma
[...] Read more.
Store-operated Ca
2+ entry (SOCE) is a ubiquitous mechanism regulating extracellular Ca
2+ entry to control a multitude of Ca
2+-dependent signaling pathways and cellular processes. SOCE relies on the concerted activity of the reticular Ca
2+ sensor STIM1 and the plasma membrane Ca
2+ channel ORAI1, and dysfunctions of these key factors result in human pathologies.
STIM1 and
ORAI1 gain-of-function (GoF) mutations induce excessive Ca
2+ influx through SOCE over-activation, and cause tubular aggregate myopathy (TAM) and Stormorken syndrome (STRMK), two overlapping disorders characterized by muscle weakness and additional multi-systemic signs affecting growth, platelets, spleen, skin, and intellectual abilities. In order to investigate the pathophysiological effect of overactive SOCE on muscle function and structure, we combined transcriptomics with morphological and functional studies on a TAM/STRMK mouse model. Muscles from
Stim1R304W/+ mice displayed aberrant expression profiles of genes implicated in Ca
2+ handling and excitation-contraction coupling (ECC), and in vivo investigations evidenced delayed muscle contraction and relaxation kinetics. We also identified signs of reticular stress and abnormal mitochondrial activity, and histological and respirometric analyses on muscle samples revealed enhanced myofiber degeneration associated with reduced mitochondrial respiration. Taken together, we uncovered a molecular disease signature and deciphered the pathomechanism underlying the functional and structural muscle anomalies characterizing TAM/STRMK.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
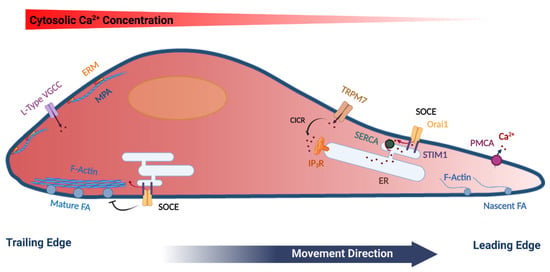
Store Operated Calcium Entry in Cell Migration and Cancer Metastasis
by
Ayat S. Hammad and Khaled Machaca
Cited by 27 | Viewed by 5364
Abstract
Ca
2+ signaling is ubiquitous in eukaryotic cells and modulates many cellular events including cell migration. Directional cell migration requires the polarization of both signaling and structural elements. This polarization is reflected in various Ca
2+ signaling pathways that impinge on cell movement.
[...] Read more.
Ca
2+ signaling is ubiquitous in eukaryotic cells and modulates many cellular events including cell migration. Directional cell migration requires the polarization of both signaling and structural elements. This polarization is reflected in various Ca
2+ signaling pathways that impinge on cell movement. In particular, store-operated Ca
2+ entry (SOCE) plays important roles in regulating cell movement at both the front and rear of migrating cells. SOCE represents a predominant Ca
2+ influx pathway in non-excitable cells, which are the primary migrating cells in multicellular organisms. In this review, we summarize the role of Ca
2+ signaling in cell migration with a focus on SOCE and its diverse functions in migrating cells and cancer metastasis. SOCE has been implicated in regulating focal adhesion turnover in a polarized fashion and the mechanisms involved are beginning to be elucidated. However, SOCE is also involved is other aspects of cell migration with a less well-defined mechanistic understanding. Therefore, much remains to be learned regarding the role and regulation of SOCE in migrating cells.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
1. Author: Dr. Raz Palty;
Tentative title: On the Involvement of SARAF in CRAC Channel Regulation
2. Author: Dr. Rodrigo S. Lacruz;
Tentative title: Linking SOCE with other Channels and Mitochondrial Function
3. Author: Prof. Khaled Machaca;
Tentative title: Role of STIM1 phosphorylation in regulating store operated Ca entry function
4. Author: Prof. Menahem Segal
Tentative title: STIMs and Orai in central neurons